- Record: found
- Abstract: found
- Article: not found
Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI
Abstract
Background/Objectives
Excessive infant weight gain in the first 6-months of life is a powerful predictor of childhood obesity and related health risks. In mice, omega-6 fatty acids (FA) serve as potent ligands driving adipogenesis during early development. The ratio of omega-6 relative to omega-3 (n-6/n-3) FA in human milk (HM) has increased 3-fold over the last 30 years, but the impact of this shift on infant adipose development remains undetermined. This study investigated how maternal obesity and maternal dietary FA (as reflected in maternal red blood cells (RBC) composition) influenced HM n-6 and n-3 FAs, and whether the HM n-6/n-3 ratio was associated with changes in infant adipose deposition between 2-weeks and 4-months postpartum.
Subjects/Methods
Forty-eight infants from normal-weight (NW), overweight (OW) and obese (OB) mothers were exclusively or predominantly breastfed over the first 4 months of lactation. Mid-feed HM and maternal RBC were collected at either transitional (2-weeks) or established (4-months) lactation, along with infant body composition assessed using air-displacement plethysmography. The FA composition of HM and maternal RBC was measured quantitatively by lipid mass spectrometry.
Results
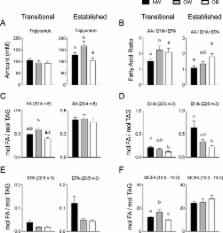
In transitional and established HM, DHA was lower ( P=0.008; 0.005) and the AA/DHA+EPA ratio was higher ( P=0.05; 0.02) in the OB relative to the NW group. Maternal prepregnancy BMI and AA/ DHA+EPA ratios in transitional and established HM were moderately correlated ( P=0.018; 0.001). Total infant fat mass was increased in the upper AA/DHA+EPA tertile of established HM relative to the lower tertile ( P=0.019). The amount of changes in infant fat mass and % body fat were predicted by AA/EPA+DHA ratios in established HM (P=0.038; 0.010).
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c.

- Record: found
- Abstract: found
- Article: found
