- Record: found
- Abstract: found
- Article: found
A Novel Peptide Oligomer of Bacitracin Induces M1 Macrophage Polarization by Facilitating Ca 2+ Influx
Read this article at
Abstract
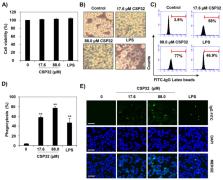
Antimicrobial peptides (AMPs) are components of the innate immune system and form the first defense against pathogens for various organisms. In the present study, we assessed whether CSP32, a novel AMP oligomer of bacitracin isolated from a strain of Bacillus spp., regulates the polarization of murine macrophage-like RAW 264.7 cells. CSP32 stimulated phagocytosis while inducing the appearance of the typical M1 polarized macrophage phenotype; these M1 macrophages play a role in host defense against pathogens. Furthermore, our results showed that CSP32 enhanced the expression and production of pro-inflammatory mediators, such as cytokines and chemokines. In addition, the CSP32-stimulated inflammatory mediators were induced mainly by the mitogen-activated protein kinase/nuclear factor kappa B (MAPK/NF-κB) signaling pathway during M1 macrophage polarization. In particular, CSP32 markedly increased the numbers of Ca 2+-positive macrophages while upregulating phospholipase C and activating protein kinase Cε. Furthermore, the inhibition of intracellular Ca 2+ by BAPTA-AM, a Ca 2+ chelator, significantly suppressed the CSP32-mediated phagocytosis, inflammatory mediator production, and NF-κB activation. In conclusion, our data suggested that CSP32-stimulated M1 macrophage polarization is dependent on the calcium signaling pathway and may result in enhanced immune capacities.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Macrophage plasticity, polarization, and function in health and disease.
- Record: found
- Abstract: found
- Article: not found