- Record: found
- Abstract: found
- Article: found
Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells

Read this article at
Summary
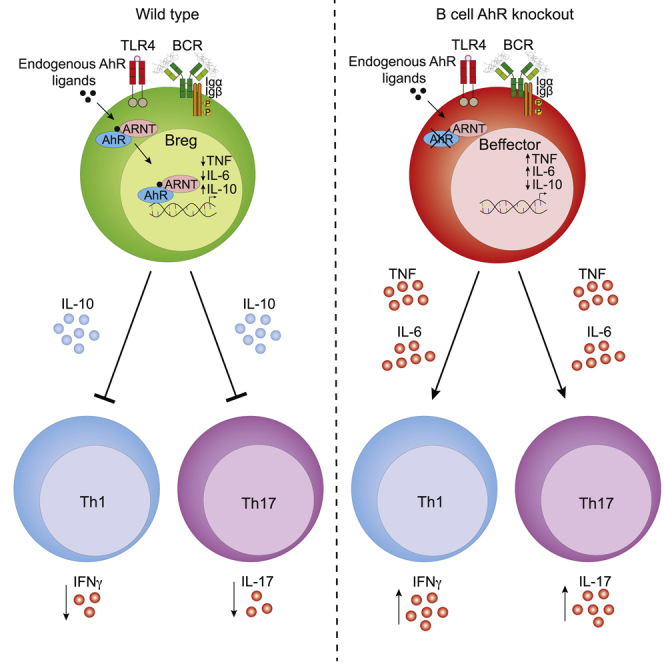
Regulatory B cells (Bregs) play a critical role in the control of autoimmunity and inflammation. IL-10 production is the hallmark for the identification of Bregs. However, the molecular determinants that regulate the transcription of IL-10 and control the Breg developmental program remain unknown. Here, we demonstrate that aryl hydrocarbon receptor (AhR) regulates the differentiation and function of IL-10-producing CD19 +CD21 hiCD24 hiBregs and limits their differentiation into B cells that contribute to inflammation. Chromatin profiling and transcriptome analyses show that loss of AhR in B cells reduces expression of IL-10 by skewing the differentiation of CD19 +CD21 hiCD24 hiB cells into a pro-inflammatory program, under Breg-inducing conditions. B cell AhR-deficient mice develop exacerbated arthritis, show significant reductions in IL-10-producing Bregs and regulatory T cells, and show an increase in T helper (Th) 1 and Th17 cells compared with B cell AhR-sufficient mice. Thus, we identify AhR as a relevant contributor to the transcriptional regulation of Breg differentiation.
Graphical Abstract
Highlights
Abstract
The transcriptional control of interleukin-10 (IL-10) in regulatory B cells (Bregs) remains undefined. Piper et al. identify the aryl hydrocarbon receptor (AhR) as an important transcription factor involved in Breg differentiation and show a direct role of AhR in the regulation of IL-10 transcription.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Small-sample estimation of negative binomial dispersion, with applications to SAGE data.
- Record: found
- Abstract: found
- Article: not found
Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease.
- Record: found
- Abstract: found
- Article: found