- Record: found
- Abstract: found
- Article: found
Siglec-H protects from virus-triggered severe systemic autoimmunity
Read this article at
Abstract
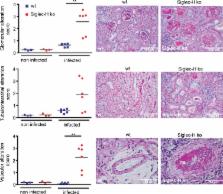
Siglec-H is a key negative regulator of the type I interferon pathway, reducing the incidence of autoimmunity after viral infection.
Abstract
It is controversial whether virus infections can contribute to the development of autoimmune diseases. Type I interferons (IFNs) are critical antiviral cytokines during virus infections and have also been implicated in the pathogenesis of systemic lupus erythematosus. Type I IFN is mainly produced by plasmacytoid dendritic cells (pDCs). The secretion of type I IFN of pDCs is modulated by Siglec-H, a DAP12-associated receptor on pDCs. In this study, we show that Siglec-H–deficient pDCs produce more of the type I IFN, IFN-α, in vitro and that Siglec-H knockout (KO) mice produce more IFN-α after murine cytomegalovirus (mCMV) infection in vivo. This did not impact control of viral replication. Remarkably, several weeks after a single mCMV infection, Siglec-H KO mice developed a severe form of systemic lupus–like autoimmune disease with strong kidney nephritis. In contrast, uninfected aging Siglec-H KO mice developed a mild form of systemic autoimmunity. The induction of systemic autoimmune disease after virus infection in Siglec-H KO mice was accompanied by a type I IFN signature and fully dependent on type I IFN signaling. These results show that Siglec-H normally serves as a modulator of type I IFN responses after infection with a persistent virus and thereby prevents induction of autoimmune disease.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus.
- Record: found
- Abstract: found
- Article: not found
Plasmacytoid dendritic cells in immunity.
- Record: found
- Abstract: found
- Article: not found