- Record: found
- Abstract: found
- Article: not found
Factors Affecting Canagliflozin-Induced Transient Urine Volume Increase in Patients with Type 2 Diabetes Mellitus
Read this article at
Abstract
Introduction
Sodium glucose co-transporter 2 (SGLT2) inhibitors exhibit diuretic activity, which is a possible mechanism underlying the cardiovascular benefit of these inhibitors. However, the osmotic diuresis-induced increase in urine volume, and the risk of dehydration have been of concern with SGLT2 inhibitor treatment. This study aimed to investigate the mechanism underlying SGLT2 inhibitor canagliflozin-induced diuresis in Japanese type 2 diabetes mellitus (T2DM) patients.
Methods
Thirteen T2DM patients received a daily oral dose of 100 mg canagliflozin before breakfast for 6 days. Blood and urine samples were collected at predetermined time points. The primary endpoint was evaluation of correlations between changes from baseline in urine volume and factors that are known to affect urine volume and between actual urine volume and these factors.
Results
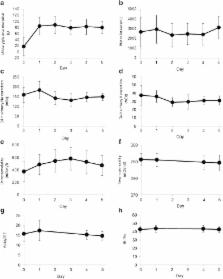
Canagliflozin transiently increased urine volume and urinary sodium excretion on Day 1 with a return to baseline levels thereafter. Canagliflozin administration increased urinary glucose excretion, which was sustained during repeated-dose administration. Plasma atrial natriuretic peptide (ANP) and N-terminal pro-b-type natriuretic peptide (NT-proBNP) levels decreased, while plasma renin activity increased. On Day 1 of treatment, changes in sodium and potassium excretion were closely correlated with changes in urine output. A post hoc multiple regression analysis showed changes in sodium excretion and water intake as factors that affected urine volume change at Day 1. Furthermore, relative to that at baseline, canagliflozin decreased blood glucose throughout the day and increased plasma total GLP-1 after breakfast.
Conclusion
Canagliflozin induced transient sodium excretion and did not induce water intake at Day 1; hence, natriuresis rather than glucose-induced osmotic diuresis may be a major factor involved in the canagliflozin-induced transient increase in urine output. In addition, canagliflozin decreased plasma ANP and NT-proBNP levels and increased plasma renin activity, which may be a compensatory mechanism for sodium retention, leading to subsequent urine output recovery.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future.
- Record: found
- Abstract: found
- Article: not found
Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications
- Record: found
- Abstract: found
- Article: not found
