- Record: found
- Abstract: found
- Article: found
Octreotide-Resistant Acromegaly: Challenges and Solutions
Abstract
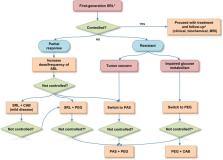
Acromegaly is a rare and severe disease caused by an increased and autonomous secretion of growth hormone (GH), thus resulting in high circulating levels of insulin-like growth factor 1 (IGF-1). Comorbidities and mortality rate are closely related to the disease duration. However, in most cases achieving biochemical control means reducing or even normalizing mortality and restoring normal life expectancy. Current treatment for acromegaly includes neurosurgery, radiotherapy and medical therapy. Transsphenoidal surgery often represents the recommended first-line treatment. First-generation somatostatin receptor ligands (SRLs) are the drug of choice in patients with persistent disease after surgery and are suggested as first-line treatment for those ineligible for surgery. However, only about half of patients treated with octreotide (or lanreotide) achieve biochemical control. Other available drugs approved for clinical use are the second-generation SRL pasireotide, the dopamine agonist cabergoline, and the GH-receptor antagonist pegvisomant. In the present paper, we revised the current literature about the management of acromegaly, aiming to highlight the most relevant and recent therapeutic strategies proposed for patients resistant to first-line medical therapy. Furthermore, we discussed the potential molecular mechanisms involved in the variable response to first-generation SRLs. Due to the availability of different medical therapies, the choice for the most appropriate drug can be currently based also on the peculiar clinical characteristics of each patient.
Most cited references124
- Record: found
- Abstract: found
- Article: not found
Acromegaly: an endocrine society clinical practice guideline.
- Record: found
- Abstract: found
- Article: not found