- Record: found
- Abstract: found
- Article: found
Dipeptidyl peptidase-4 inhibitor linagliptin attenuates neointima formation after vascular injury
Read this article at
Abstract
Background
Recently, glucagon-like peptide-1 (GLP-1)-based therapy, including dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists, has emerged as one of the most popular anti-diabetic therapies. Furthermore, GLP-1-based therapy has attracted increased attention not only for its glucose-lowering ability, but also for its potential as a tissue-protective therapy. In this study, we investigated the vascular-protective effect of the DPP-4 inhibitor, linagliptin, using vascular smooth muscle cells (VSMCs).
Methods
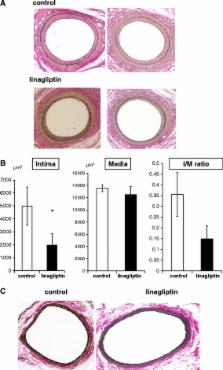
Six-week-old male C57BL/6 mice were divided into control (n =19) and linagliptin (3 mg/kg/day, n =20) treated groups. Endothelial denudation injuries were induced in the femoral artery at 8 weeks of age, followed by evaluation of neointima formation at 12 weeks. To evaluate cell proliferation of rat aortic smooth muscle cells, a bromodeoxyuridine (BrdU) incorporation assay was performed.
Results
Linagliptin treatment reduced vascular injury-induced neointima formation, compared with controls ( p <0.05). In these non-diabetic mice, the body weight and blood glucose levels did not change after treatment with linagliptin. Linagliptin caused an approximately 1.5-fold increase in serum active GLP-1 concentration, compared with controls. In addition, the vascular injury-induced increase in the oxidative stress marker, urinary 8-OHdG, was attenuated by linagliptin treatment, though this attenuation was not statistically significant ( p =0.064). Moreover, linagliptin did not change the serum stromal cell-derived factor-1α (SDF-1α) or the serum platelet-derived growth factor (PDGF) concentration. However, linagliptin significantly reduced in vitro VSMC proliferation.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease- associated Aβ oligomers.
- Record: found
- Abstract: found
- Article: not found
Reactive oxygen species in the vasculature: molecular and cellular mechanisms.
- Record: found
- Abstract: found
- Article: found