- Record: found
- Abstract: found
- Article: found
A Randomized, Open-Label, Bioequivalence Study of Lidocaine Topical System 1.8% and Lidocaine Patch 5% in Healthy Subjects
Abstract
Purpose
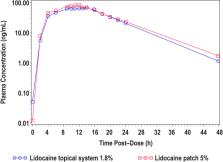
This study was designed to characterize drug delivery with lidocaine topical system 1.8% vs lidocaine patch 5% through 2 PK studies.
Patients and Methods
Two Phase 1, single-center, open-label, randomized PK studies were performed in healthy adults. In Study 1, 56 subjects received a single intravenous bolus of 0.7 mg/kg of lidocaine as a lead-in to allow for the accurate determination of apparent dose of both products. After a 7-day washout period, subjects were randomized to receive either lidocaine topical system 1.8% or lidocaine patch 5% for 12 hours followed by another 7-day washout period, after which subjects crossed over to receive the other treatment for 12 hours. In Study 2, 54 subjects were randomized to receive either lidocaine topical system 1.8% or lidocaine patch 5% for 12 hours. After a 7-day washout period, subjects crossed over to receive the other treatment. Adhesion and skin irritation assessments were performed after application of the products in Study 2. In both studies, serial blood samples were collected to measure the plasma concentration of lidocaine after product application. Safety assessments and adverse events were monitored in both studies.
Results
The comparative PK analysis demonstrated that the two products, despite their difference in drug load and strength, are bioequivalent. Both products were well tolerated. In Study 2, dermal response scores (skin tolerability after removal) were similar between lidocaine topical system 1.8% and lidocaine patch 5%, with a mean irritation score per patch <1 (barely perceptible erythema), which is not considered to be clinically significant.
Conclusion
Bioequivalence was demonstrated between lidocaine topical system 1.8% and lidocaine patch 5%. A comparison of the single-time adhesion scores at 12 hours in Study 2 favored lidocaine topical system 1.8% over lidocaine patch 5%. Both products were well tolerated as a single application in healthy adult human subjects.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Post-herpetic Neuralgia: a Review.
- Record: found
- Abstract: found
- Article: not found
Topical therapies in the management of chronic pain.

- Record: found
- Abstract: found
- Article: found
