- Record: found
- Abstract: found
- Article: found
Apatinib for molecular targeted therapy in tumor
Abstract
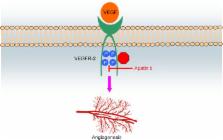
As tumor angiogenesis is one of the hallmarks of cancer, the inhibition of vascular endothelial growth factor signaling has become an attractive anticancer approach. Apatinib, a small-molecule inhibitor of vascular endothelial growth factor receptor-2, has demonstrated encouraging anticancer activity across a broad range of malignancies, including gastric cancer, non-small-cell lung cancer, breast cancer, and hepatocellular carcinoma. In this up-to-date review, focus is not only on the structure, mechanisms, and pharmacokinetics of apatinib, but also on summarizing clinical trials and making recommendations of apatinib for patients with advanced solid tumors.
Most cited references29
- Record: found
- Abstract: found
- Article: not found
YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo.
- Record: found
- Abstract: found
- Article: not found
Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer.
- Record: found
- Abstract: found
- Article: not found