- Record: found
- Abstract: found
- Article: found
A multi-centre randomised controlled study of pre-IVF outpatient hysteroscopy in women with recurrent IVF implantation failure: Trial of Outpatient Hysteroscopy - [TROPHY] in IVF
Read this article at
Abstract
Background
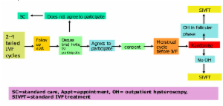
The success rate of IVF treatment is low. A recent systematic review and meta-analysis found that the outcome of IVF treatment could be improved in patients who have experienced recurrent implantation failure if an outpatient hysteroscopy (OH) is performed before starting the new treatment cycle. However, the trials were of variable quality, leading to a call for a large and high-quality randomised trial. This protocol describes a multi-centre randomised controlled trial to test the hypothesis that performing an OH prior to starting an IVF cycle improves the live birth rate of the subsequent IVF cycle in women who have experienced two to four failed IVF cycles.
Methods and design
Eligible and consenting women will be randomised to either OH or no OH using an internet based trial management programme that ensures allocation concealment and employs minimisation for important stratification variables including age, body mass index, basal follicle stimulating hormone level and number of previous failed IVF cycles. The primary outcome is live birth rate per IVF cycle started. Other outcomes include implantation, clinical pregnancy and miscarriage rates.
The sample size for this study has been estimated as 758 participants with 379 participants in each arm. Interim analysis will be conducted by an independent Data Monitoring Committee (DMC), and final analysis will be by intention to treat. A favourable ethical opinion has been obtained (REC reference: 09/H0804/32).
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials.
- Record: found
- Abstract: found
- Article: not found
Women's emotional adjustment to IVF: a systematic review of 25 years of research.
- Record: found
- Abstract: found
- Article: not found