INTRODUCTION
The increasing interest to the alkaliphilic Bacillus sp. is in connection with a great impact that these alkaliphilic microorganisms have gotten by their valuable and commercially important enzymes [1–3]. Various applications, e.g., in detergents [4], dye decolorization [5], pulp and paper industry [6, 7], have prompted the isolation of strains from a variety of alkaline environments as a source of enzymes with suitable activities.
The alkaliphilic Bacilli are the best producers of the enzyme cyclodextrin glucanotransferase (CGTase, EC 2.4.1.19). Cyclodextrin glucanotransferase (CGTase, EC 2.4.1.9) is a unique enzyme capable of converting starch and related substrates into cyclodextrins (CDs) [8]. CDs are cyclic ring structure compounds consisting of six, seven, or eight glucose residues joined by α(1→4) linkages (α-, β-, and γ-CD, respectively) [9]. These compounds have an exclusive ability to act as molecular containers by entrapping hydrophobic molecules in their internal cavity. This property can improve stability, solubility, and availability of a wide variety of interesting compounds used in pharmaceutical, food, cosmetic, agricultural, and chemical industries [10–13].
Besides the ability of CGTases to catalyze the intramolecular transglycosylation reaction (cyclization), they are also able to perform two intermolecular transglycosylation reactions: coupling, in which a CD ring is cleaved and transferred to a linear acceptor substrate and disproportionation, wherein two linear oligosaccharides are converted into linear oligosaccharides of different sizes. In addition, these enzymes possess a weak hydrolyzing activity in which water is the glycosyl acceptor [14, 15].
The industrial production of CGTase was made attractive only when alkaliphilic Bacillus sp. was introduced as a production organism [16]. The separation of the different CDs is costly and time-consuming. A CGTase that synthesizes predominantly one type of CD is of interest [17]. The majority of the CGTases especially from the alkaliphilic bacteria convert starch into β-CD as the main product but still in a mixture of CDs of different ratios [18].
The obtaining of new CGTases has a great scientific and practical contribution to the enzyme production of CDs [19]. In this study, we report the purification and characterization of the CGTase produced by the alkaliphilic Bacillus sp. B3 strain isolated by us.
MATERIALS AND METHODS
Material
α-, β-, and γ-CD and Sephadex G-50 were purchased from Pharmacia Biotech. Soluble starch, yeast extract and peptone were purchased from Sigma. Phenolphthalein was purchased from Merck. All of the chemicals were of analytical grade.
Screening and isolation of bacteria
Soil samples of 1 g were suspended in 10 mL of sterilized water; 0.1 mL of the suspensions were inoculated in plates containing agar Horikoshi II containing the following (in w/v): 1.0% soluble starch, 0.5% yeast extract, 0.5% peptone, 0.1% KH2PO4, 0.02% MgSO4·7H2O, 0.02% phenolphthalein and 1.0% Na2CO3 (autoclave separately). Medium was incubated at 37°C. Plates were incubated at 37°C for 24 h. Bacterial colonies which produced the largest clear halo zones were selected for further studies [20].
CGTase production and purification
The selected strain was cultivated in flasks containing 200 mL of Horikoshi II broth culture medium and incubated at 37°C for 48 h at 200 rpm [21]; 500 ml of culture medium was across diatomaceous earth. The resulting liquid volume was concentrated with rotoevaporation at 30°C. The extract was centrifugated at 3000 rpm for 15 min and applied to Sephadex chromatography gel G-50 (15 × 3.76) with mobile phase buffer Tris/HCl pH 8.0 20 mM. The protein concentration was estimated for Bradford method [22].
Cyclizing activity of CGTase
The cyclizing activity of CGTase was determined according to the phenolphthalein method, measuring spectrophotometrically at 550 nm the production of β-CD on the basis of its ability to form a colourness inclusion complex with this dye. Phenolphthalein (4 mM) in ethanol is diluted in 125 mM Na2CO3 pH 11 just before starting the assay; 1% starch in 0.05 M citrate-phosphate buffer pH 5.0 was used as substrate. One unit of CGTase is defined as the amount of enzyme catalyzing the production of 1 μmol of β-CD per minute under the reaction conditions [23, 24].
Optimum pH
The cyclizing activity of CGTase was measured at 30°C for 10 min in the following buffers: 100 mM sodium acetate/acetic acid, pH 3.0–5.5; 100 mM K2HPO4/KH2PO4, pH 6.0–7.5; 100 mM Tris/HCl, pH 8.0–9.0; and Na2CO3/NaHCO3, pH 9.5–11.5.
pH stability
Enzyme preparations (0.57 U) were incubated at 4°C in the following buffers: 100 mM sodium acetate/acetic acid, pH 3.0–5.5; 100 mM K2HPO4/KH2PO4, pH 6.0–7.5; 100 mM Tris/HCl, pH 8.0–9.0; and Na2CO3/NaHCO3, pH 9.5–11.5. Aliquots were removed after 24 h of incubation, diluted in 0.1 M K2HPO4/ KH2PO4 buffer, pH 7.0, and assayed for cyclizing activity of CGTase.
Thermostability
Enzyme preparations (0.57 U) were incubated at different temperatures between 35°C and 75°C in 100 mM potassium phosphate buffer, pH 7.0. Aliquots were removed after 30 min of incubation, chilled quickly, and assayed for cyclizing activity.
Optimum temperature
The activity of enzyme preparations was measured at different temperatures ranging from 30°C to 80°C; after 10 min, incubation and assay process for cyclizing activity was done.
Determination of kinetic parameters
To determine the Michaelis–Menten constant (Km), the activity assay was applied for different substrate (starch) concentrations. Starch solutions (40–0.31 g/L) were prepared in potassium phosphate buffer, 0.1 mol/l (pH 7.0) and kept in a water bath at 60°C for 10 min, and then the enzyme solution was added to the test tubes and shaken for different incubation times. The dates were adjusted to the substrate inhibition model using the software Gradpad Prism.
Production of different CDs
The enzyme (0.57 U) was incubated for 300 min with 5% starch in potassium phosphate buffer (pH = 7, 100 mM) at 50°C for 5 h. The concentration of different CDs produced by the action of the purified CGTase on soluble starch was determined by colorimetric methods. The concentration of α-CD was assayed by the decrease in absorbance at 507 nm due to formation of a methyl orange–α-CD complex [25]. The concentration of β-CD was determined according to the method described above [23, 24]. The concentration of γ-CD was determined by measuring the absorbance at 630 nm due to the formation of an inclusion complex with bromocresol green [26].
RESULTS AND DISCUSSION
CGTase was purified partially by rotoevaporation and chromatography on Sephadex G-50. The rotoevaporation step was used to concentrate the enzyme preparation. Figure 1 shows a chromatography which was obtained after rotoevaporation step. The CGTase was eluted as one peak containing the highest protein content and enzymatic activity of all fractions (Figure 1). Therefore, further experiments of the characterization of this enzyme were carried out with fraction to 10–20 of chromatography. The enzyme could be sufficiently purified using two-step purification with a yield of 66.5% activity and 1.49-fold purification for specific enzymatic activity of 5.2 U/mg (one unit of enzyme activity is defined as μmoles of β-CD per minute under the reaction condition; Table 1). The yield of this purification was better than the one obtained by Bejar et al. [8], Tachibana et al. [27], and similar to Tonkova et al. [28] for CGTases isolated of Paenibacillus pabuli, Archaeon, a Thermococcus sp. and Bacillus pseudalcaliphilus respectively. This method of purification is suitable for elimination of CD present in the culture medium that interferes in the activity assay.

| Purification steps | Total volume (mL) | Enzymatic activity (U/mL) | Protein concentration (mg/mL) | Specific activity (U/mg) | Purification fold | Yield (%) |
|---|---|---|---|---|---|---|
| Crude enzyme before rotoevaporation | 500 | 0.16 | 0.12 | 1.3 | 1 | 100.0 |
| Eluate after chromatography on Sephadex G-50 | 40 | 1.3 | 0.25 | 5.2 | 1.49 | 66.5 |
The effect of pH on CGTase activity was established by determination of the enzyme activity at varying pH value ranging from 3.0 to 11.5 at 30°C (Figure 2). The purified enzyme exhibited two pH peaks at pH 6.2 and 7.1, similar to CGTase from Bacillus lentus [29]. The establishment of two pH optima may been caused by the subtle structural change due to alkaline pH [28]. However literature from different authors reported that CGTases from Bacillus sp. show an optimal cyclizing activity in pH range from 5.0 to 10.0 [30, 31, 32].
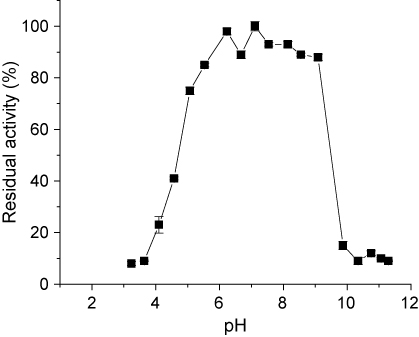
The pH stability of the enzyme was determined by preincubating enzyme in buffers between 3.0 and 11.5 pH values during 24 h at 4°C, and then residual activities were measured under standard assay conditions (Figure 3). The CGTase obtained was highly stable at pH 5.0 and 11.0 maintaining 84% and 80% residual activities, respectively. This property permit to apply this enzyme in a wide range of pH.
The heat stability of CGTase was evaluated from the activity retained after heating the enzyme at different temperatures for 30 min. A temperature activity profile on enzyme showed increasing enzyme activity up to 45°C and which decreased thereafter (Figure 4). The T50 value – defined as the temperature at which 50% of the initial activity was retained – was the 63°C.
The activities of enzyme were measured at the temperature in the range 30–80°C with soluble starch as substrate. Figure 5 shows the enzyme to be optimally active at 60°C. This result was the best obtained among other CGTases from Bacillus megaterium [33], Bacillus macerans IFO 3490 [34] and B. lentus [35].
The Vmax and Km values obtained by starch as substrate were 5.2 μM β-CD/min ml and 4.1 g/L, respectively. Considering that the Km parameter is correlated to the affinity of the enzyme for its substrate, the value observed in this work indicates that the enzyme has comparatively higher affinity for the substrate. In the literature, some Km values measured for CGTase are higher than the value obtained in this work for example: Bacillus circulans E 192, 5.7 g/L [36] and Bacillus agaradhaerens 21.2 g/L [37]. All the values shown above, obtained by different researchers, were based on soluble starch as the substrate. The substrate inhibition constant Ks gave 26.8 L/g.
The production yield and ratio of the different CDs formed by CGTases are dependent not only on the microbial source producing the enzyme but also on the nature of the substrate and the bioconversion conditions (such as temperature, pH, and time). The profile of CDs production with time, during the action of Bacillus sp. CGTase, was shown in Figure 6. A maximum conversion was obtained with 2 U enzyme/g of substrate after 3 h of reaction. At this point, the CGTase generated a mixture of CDs in the ratio of 1:0.43:0.94 for α-, β-, and γ-CD, respectively.

It should be noted that the CGTase from Bacillus sp. can be used for an efficient production of CDs without any additives. In many reports, some additives, such as toluene [38], Ca2+ and polyols [39], or pullulanase pretreatment of starch [38], were applied to increase the CD production. Specific applications in food and pharmaceutical industries require CDs completely free of these toxic (in most cases) agents. Complete removal of these compounds is often difficult and expensive.
Therefore, the CGTase from Bacillus sp. which gave a significant yield of γ-CDs in comparison to recombinant CGTase from alkaliphilic Bacillus sp. TS1 [40] without using any additives could be of interest for their industrial production.
CONCLUSIONS
In this study, the purification and characterization of a novel CGTase from Bacillus sp. isolated from Cuba soil were reported. The purification of the enzyme was achieved by chromatography and recovery of 66.5% activity and 4-fold purification. This enzyme could be effectively used for conversion starch into CD in a wide pH range from 5.0 to 9.0. Besides retains an 80% of the residual activity after incubated a pH = 11 during 24 h. The enzyme exhibits a good thermostability and shows temperature optimum at 60°C. The purified CGTase from Bacillus sp. could be used for an efficient CD production which is the significant yield of γ-CDs.