- Record: found
- Abstract: found
- Article: not found
Glycoprotein Biomarker Panel for Pancreatic Cancer Discovered by Quantitative Proteomics Analysis
Read this article at
Abstract

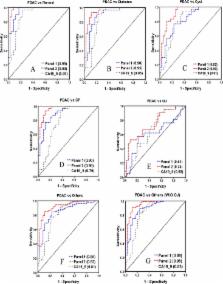
Pancreatic cancer is a lethal disease where specific early detection biomarkers would be very valuable to improve outcomes in patients. Many previous studies have compared biosamples from pancreatic cancer patients with healthy controls to find potential biomarkers. However, a range of related disease conditions can influence the performance of these putative biomarkers, including pancreatitis and diabetes. In this study, quantitative proteomics methods were applied to discover potential serum glycoprotein biomarkers that distinguish pancreatic cancer from other pancreas related conditions (diabetes, cyst, chronic pancreatitis, obstructive jaundice) and healthy controls. Aleuria aurantia lectin (AAL) was used to extract fucosylated glycoproteins and then both TMT protein-level labeling and label-free quantitative analysis were performed to analyze glycoprotein differences from 179 serum samples across the six different conditions. A total of 243 and 354 serum proteins were identified and quantified by label-free and TMT protein-level quantitative strategies, respectively. Nineteen and 25 proteins were found to show significant differences in samples between the pancreatic cancer and other conditions using the label-free and TMT strategies, respectively, with 7 proteins considered significant in both methods. Significantly different glycoproteins were further validated by lectin-ELISA and ELISA assays. Four candidates were identified as potential markers with profiles found to be highly complementary with CA 19–9 ( p < 0.001). Obstructive jaundice (OJ) was found to have a significant impact on the performance of every marker protein, including CA 19–9. The combination of α-1-antichymotrypsin (AACT), thrombospondin-1 (THBS1), and haptoglobin (HPT) outperformed CA 19–9 in distinguishing pancreatic cancer from normal controls (AUC = 0.95), diabetes (AUC = 0.89), cyst (AUC = 0.82), and chronic pancreatitis (AUC = 0.90). A marker panel of AACT, THBS1, HPT, and CA 19–9 showed a high diagnostic potential in distinguishing pancreatic cancer from other conditions with OJ (AUC = 0.92) or without OJ (AUC = 0.95).
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies.
- Record: found
- Abstract: found
- Article: not found
Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database.
- Record: found
- Abstract: found
- Article: not found
