- Record: found
- Abstract: found
- Article: found
Clinical application of lying-on-the-floor total skin electron irradiation for frail patients with cutaneous lymphoma: An emphasis on the importance of in vivo dosimetry
brief-report
Jaden D. Evans , MD
a ,
Laura L. Haley , BS
b ,
Sarah E. Locher , PhD
a ,
Michael P. Grams , PhD
a ,
Christopher L. Deufel , PhD
a ,
John A. Antolak , PhD
a ,
James A. Martenson , MD
a
,
∗
05 April 2016
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Total skin electron irradiation (TSEI) is an effective option for cutaneous T-cell
lymphoma (CTCL).1, 2 Two conventional methods used to deliver TSEI are the Stanford
multiple dual field technique and the McGill rotational technique3, 4; however, both
techniques require patients to stand for 10 to 30 minutes and cannot be used in nonambulatory
patients. Our group has previously described technical parameters for “lying-on-the-floor”
total skin electron beam therapy for nonambulatory patients.
5
We now report clinical implementation of this technique in a nonambulatory patient
with progressive CTCL with particular emphasis on the critical importance of in vivo
dosimetry.
Case presentation
A 67-year-old male with progressive CTCL was seen for consideration of palliative
radiation therapy 14 months after his original diagnosis. Symptoms and findings at
the time of diagnosis had included an intensely pruritic, diffuse, erythematous truncal
rash. Multiple biopsies demonstrated atypical dermatotropic lymphoid infiltrate consistent
with CTCL. He had received 3 different courses of systemic therapy including: cyclophosphamide,
hydroxydaunorubicin, vincristine, and prednisone chemotherapy; romidepsin; and bexarotene
before presenting for radiation therapy. He presented with extensive disease, including
multiple ulcerative lesions (Fig 1). The plantar surfaces of his feet were spared
by his disease process. He was treated initially with a course of conventional standing
TSEI without supplemental irradiation to the soles of the feet, 16 Gy in 4 fractions
given approximately every 7 to 10 days.
6
Figure 1
Patient's initial presentation to radiation oncology after 3 different courses of
systemic therapy and before total skin electron irradiation.
Initial TSEI was well tolerated; 2 months later, he had near complete response (Fig
2) of his cutaneous disease except for the soles of his feet where he had progression.
A total of 8 Gy in 2 fractions to this area resulted in complete response. He was
then started on bendamustine and methylprednisolone after further workup revealed
innumerable pulmonary nodules histologically confirmed to be T-cell lymphoma.
Figure 2
Patient 2 months after total skin electron irradiation.
Approximately 4 weeks later, he returned for re-evaluation with extensive cutaneous
recurrence. His pulmonary disease showed interval improvement, suggesting a mixed
response to the bendamustine and methylprednisolone. His overall condition had deteriorated
and he was no longer able to stand for treatment. As such, a lying-on-the-floor TSEI
technique was recommended. He was treated using this technique with an additional
12 Gy in 3 fractions, in which each fraction was delivered every 7 to 10 days (minor
variation resulted from patient and physician availability). His cutaneous disease
was well controlled with results similar to Fig 2. Two additional brief courses of
single fraction (4 Gy) TSEI were sufficient to maintain skin integrity until the patient
died of respiratory failure resulting from progressive pulmonary involvement by his
lymphoma 6 months after his initial radiation oncology consultation.
Lying-on-the-floor TSEI technique
Other investigators have described techniques for total skin electron beam therapy
for nonambulatory patients.4, 7 A major modification in our technique is the use of
a customized copper flattening filter to improve treatment field uniformity, which
eliminates the need for field junctioning and minimizes setup time.
5
This technique also does not require match lines. Treatment was delivered with 6-MeV
electrons. A polycarbonate spoiler (2 m × 1 m × 4 mm) was used for electron scatter
and beam energy degradation.
The patient setup is depicted in Figs 3 and 4. The 6 conventional standing positions1,
4 are reproduced on the floor and described here. For the anteroposterior (AP) and
posteroanterior (PA) positions, the patient's umbilicus is positioned either supine
or prone directly below the isocenter with the skin surface about 5 cm below the polycarbonate
spoiler, and the patient is oriented perpendicular to the LINAC waveguide. The patient
lies on a thin mattress (about 3 cm thick) with arms and legs partially away from
the body and fingers spread apart. Three gantry angles of 0°, 60°, and 300° were used
to provide optimal dose homogeneity for both the AP and PA positions. Monitor unit
(MU) weighting for the gantry angles, which were empirically determined and described
previously,
5
were MU300° equal to MU60°, and MU0° equal to 0.41 MU60° to account for the fact that
the MU0° delivers more dose per MU than the 60° and 300° beams. The left posterior
oblique, right posterior oblique, left anterior oblique, and right anterior oblique
positions are set up with the patient oriented parallel to the waveguide and the umbilicus
at a distance of 230 cm from the isocenter, with a gantry setting of 300°. The polycarbonate
spoiler is positioned adjacent to the patient as depicted in Fig 4.
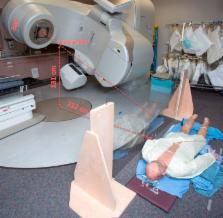
Figure 3
Lying-on-the-floor total skin electron irradiation setup with the customized flattening
filter technique. (A) Radiochromic film was used at various anatomical locations for
in vivo measurements. (B) Schematic of anteroposterior and posteroanterior treatment
fields (adapted from Deufel and Antolak
5
; used with permission). Note that only the anteroposterior setup is shown here. (C-E)
The patient is oriented perpendicular to the LINAC waveguide (prone treatment fields
not illustrated). His umbilicus was positioned directly below the central axis, 5
cm from the spoiler, and the gantry was angled to 300°, 0°, and 60°, respectively.
Figure 4
The left posterior oblique, right posterior oblique, left anterior oblique, and right
anterior oblique positions were delivered with the gantry rotated to 300° with the
patient oriented parallel to the LINAC waveguide and the umbilicus approximately 230
cm from isocenter (supine treatment fields not illustrated).
Calibration of the treatment has also been previously described.
5
In brief, a parallel plate ion chamber (Advanced Markus Type No. TN34045; PTW, Freiburg,
Germany) and solid water was used under standard reference conditions of a 10 × 10
cm2 cone size, 100 cm source–skin distance, and 1.3 cm depth to obtain a cGy/nC conversion
factor for the 6-MeV beam on high dose rate total skin electron mode. This provided
a measured dose per unit charge collected in the chamber. The chamber and solid water
was then put into a position more representative of the patient's anatomy during treatment.
In our patient's case, this consisted of putting the chamber surface about 25 cm above
the floor and the spoiler 5 cm above the chamber. Pragmatically, the chamber surface
should be at the same point as the nominal prescription point, the umbilicus. We then
use 30 × 40 cm2 fields with the custom copper filter in place to scatter the electrons
and deliver 1000 MU at gantry angles of 0°, 60°, and 300°. A similar procedure is
performed for the oblique fields. The chamber and solid water is placed behind the
spoiler again and the assembly is angled to 60° to mimic the oblique slope of the
patient's body when lying down. This setup allowed us to determine the monitor units
needed to deliver the prescription dose under ideal conditions.
A body factor, which is essentially a multiplicative factor that takes into account
the dose delivered to a point on the patient as they rotate through all of the positions,
was also incorporated as previously described.
5
For body factor measurements, radiochromic film (Gafchromic EBT3; International Specialty
Products Inc, Wayne, NJ) was affixed to the surface of the RANDO anthropomorphic phantom
(The Phantom Laboratory, Salem, NY) in 60° increments. A body factor of 3.1 was calculated
as the ratio of the summed dose delivered to a point on a standard anthropomorphic
phantom transitioned through all treatment fields depicted in Figs 3 and 4 to the
dose delivered from a single AP treatment field. However, we subsequently learned
that the body factor on the phantom was larger than the patient's in vivo body factor.
In vivo dosimetric measurements allowed adjustments of dose delivery. In the previously
published technical details of this approach, radiochromic film showed excellent agreement
with ionization chamber results, and a film calibration curve showed that the standard
deviation of dose (200 cGy delivered) was <1.3% between any given piece of film.
5
Verification of the radiochromic film's accuracy and reproducibility using the lying-on-the-floor
technique was demonstrated by previously comparing the normalized dose by anatomic
site in both the Stanford standing technique and the lying-on-the-floor technique.
5
These measurements were made by taping pieces of 2 × 2 cm2 radiochromic film onto
the patient's skin at representative locations on the head and neck, torso, and extremities.
Setup time limitations made it impractical to measure all sites for every treatment.
The radiochromic film was covered with a layer of plastic wrap so that the film itself
did not come into direct contact with the patient's skin and cleaning of the film
was not necessary before analysis. Doses obtained from in vivo measurements are presented
in Table 1. Dosimetry was obtained at the level of the umbilicus anteriorly, posteriorly,
and lateral to the left of the umbilicus at every treatment. Eighteen other sites
were sampled for 1 or more treatments. The average in vivo dose measurement was 78%
of the prescription dose of 400 cGy for the first fraction. Monitor units were cautiously
increased by 10% for the second treatment with a goal of achieving an average dose
of 90% of the prescription. Furthermore, during the second treatment, additional films
were used to measure an in vivo body factor and check the delivered dose for each
field. The body factor was calculated in vivo to be 2.7 as opposed to the 3.1 measured
with a rigid phantom, approximately 15% lower. The average in vivo dose measurement
for the second fraction was 87% of the prescribed 400 cGy. The MUs were increased
by an additional 15% for the third fraction, resulting in an average in vivo dose
measurement of 99% of the prescribed 400 cGy.
Table 1
In vivo dosimetric measurements
Location
Fraction 1 (400 cGy)
Fraction 2 (400 cGy)
Fraction 3 (400 cGy)
Dose (cGy)
% of 400 cGy
Dose (cGy)
% of 400 cGy
Dose (cGy)
% of 400 cGy
Umbilicus, anterior
320
80
363
91
396
99
Umbilicus, left anterior oblique∗
232
58
331
83
349
87
Umbilicus, right anterior oblique∗
302
76
301
75
Umbilicus, posterior
315
79
339
85
401
100
Umbilicus, left posterior oblique∗
377
94
466
117
Umbilicus, right posterior oblique∗
348
87
366
92
Upper back
359
90
421
105
Posterior neck
406
102
412
103
Right lateral shoulder
309
77
349
87
Right forearm
274
69
309
77
Left anterior thigh
328
82
Left posterior calf
348
87
395
99
Left dorsal foot
327
82
Left anterior wrist
454
114
Anterior chest
398
100
Right anterior thigh
399
100
Right lateral hip
363
91
458
115
Right anterior shin
424
106
Right posterior calf
276
69
Forehead
293
73
335
84
Average
312
78
349
87
394
99
∗
For these sites, in vivo dosimetry was obtained at the intersection of the central
axis and the patient's body for the left anterior oblique, right anterior oblique,
left posterior oblique, and right posterior oblique fields.
Discussion
In vivo dosimetry was critical for successful treatment delivery. MUs delivered were
systematically increased on progressive treatments according to the in vivo measurements
obtained with radiochromic film. Second, an extensive simulation during the patient's
initial visit was not done before the first treatment, and would have better defined
the treatment conditions. There was approximately a (1.1*1.15)/0.99 = 1.28 or 28%
discrepancy between the expected and actual doses observed in vivo. Of the 28%, we
believe 15% may be attributed to the patient-specific body factor. The remainder of
this dose discrepancy may be attributed to setup variation, which we propose could
largely be mitigated by a thorough simulation process. Specifically, the proposed
simulation would have included detailed measurements of the patient's physical dimensions
on the treatment floor. The AP/PA thickness, the lateral width, and the distance from
the patient's skin surface (both supine and prone) to the LINAC would have been useful
to accurately represent the locations to which the calibration parallel plate chamber
should be positioned. After cautiously increasing the monitor units after the first
treatment, the discrepancy between the in vivo measured dose and the expected dose
prompted further evaluation of another potential contributing factor: the body factor.
The patient-specific body factor during the actual treatment was 2.7 versus 3.1 measured
on a rigid anthropomorphic phantom. Using the phantom during the commissioning of
this technique, the estimated body factor was approximately 15% greater than the in vivo
measured factor and adjustments had to be made to the treatment setup on the second
day to account for the patient's body habitus.
Teaching case: Key learning points
This teaching case demonstrates that TSEI may be effectively used in nonambulatory
patients using a lying-on-the-floor technique. This case also shows that simulation
before the first treatment and in vivo measurements are critical for accurate delivery
of dose using this technique.
Related collections
Most cited references7
- Record: found
- Abstract: found
- Article: not found
How I treat mycosis fungoides and Sézary syndrome.
Sean Whittaker, Miles Prince, Richard T Hoppe (2009)
- Record: found
- Abstract: found
- Article: not found
Mycosis fungoides: radiation therapy.
Richard T Hoppe (2003)
- Record: found
- Abstract: found
- Article: not found
Clinical implementation of total skin electron beam (TSEB) therapy: a review of the relevant literature.
E Efstathopoulos, P Pantelakos, G Panayiotakis … (2011)