- Record: found
- Abstract: found
- Article: found
Introduction of Syphilis Point-of-Care Tests, from Pilot Study to National Programme Implementation in Zambia: A Qualitative Study of Healthcare Workers’ Perspectives on Testing, Training and Quality Assurance
Read this article at
Abstract
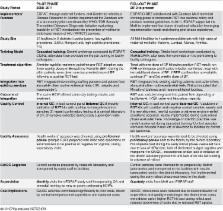
Syphilis affects 1.4 million pregnant women globally each year. Maternal syphilis causes congenital syphilis in over half of affected pregnancies, leading to early foetal loss, pregnancy complications, stillbirth and neonatal death. Syphilis is under-diagnosed in pregnant women. Point-of-care rapid syphilis tests (RST) allow for same-day treatment and address logistical barriers to testing encountered with standard Rapid Plasma Reagin testing. Recent literature emphasises successful introduction of new health technologies requires healthcare worker (HCW) acceptance, effective training, quality monitoring and robust health systems. Following a successful pilot, the Zambian Ministry of Health (MoH) adopted RST into policy, integrating them into prevention of mother-to-child transmission of HIV clinics in four underserved Zambian districts. We compare HCW experiences, including challenges encountered in scaling up from a highly supported NGO-led pilot to a large-scale MoH-led national programme. Questionnaires were administered through structured interviews of 16 HCWs in two pilot districts and 24 HCWs in two different rollout districts. Supplementary data were gathered via stakeholder interviews, clinic registers and supervisory visits. Using a conceptual framework adapted from health technology literature, we explored RST acceptance and usability. Quantitative data were analysed using descriptive statistics. Key themes in qualitative data were explored using template analysis. Overall, HCWs accepted RST as learnable, suitable, effective tools to improve antenatal services, which were usable in diverse clinical settings. Changes in training, supervision and quality monitoring models between pilot and rollout may have influenced rollout HCW acceptance and compromised testing quality. While quality monitoring was integrated into national policy and training, implementation was limited during rollout despite financial support and mentorship. We illustrate that new health technology pilot research can rapidly translate into policy change and scale-up. However, training, supervision and quality assurance models should be reviewed and strengthened as rollout of the Zambian RST programme continues.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Rapid tests for sexually transmitted infections (STIs): the way forward.

- Record: found
- Abstract: found
- Article: found
Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis
- Record: found
- Abstract: found
- Article: not found