- Record: found
- Abstract: found
- Article: found
Trans-Chalcone Attenuates Pain and Inflammation in Experimental Acute Gout Arthritis in Mice
Read this article at
Abstract
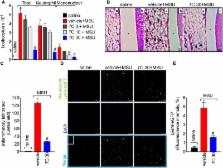
Gouty arthritis is characterized by an intense inflammatory response to monosodium urate crystals (MSU), which induces severe pain and reduction in the life quality of patients. Trans-Chalcone (1,3-diphenyl-2-propen-1-one) is a flavonoid precursor presenting biological activities such as anti-inflammatory and antioxidant proprieties. Thus, the aim of this work was to evaluate the protective effects of trans-Chalcone in experimental gout arthritis in mice. Mice were treated with trans-Chalcone (3, 10, or 30 mg/kg, per oral) or vehicle (Tween 80 20% plus saline) 30 min before intra-articular injection of MSU (100 μg/knee joint, intra-articular). We observed that trans-Chalcone inhibited MSU-induced mechanical hyperalgesia, edema, and leukocyte recruitment (total leukocytes, neutrophils, and mononuclear cells) in a dose-dependent manner. Trans-Chalcone also decreased inflammatory cell recruitment as observed in Hematoxylin and Eosin (HE) staining and the intensity of fluorescence of LysM-eGFP+ cells in the confocal microscopy. Trans-Chalcone reduced MSU-induced oxidative stress as observed by an increase in the antioxidant defense [Glutathione (GSH), Ferric Reducing (FRAP), and 2,2’-Azinobis-3-ethylbenzothiazoline 6-sulfonic acid (ABTS assays)] and reduction in reactive oxygen and nitrogen species production [superoxide anion (NBT assay) and nitrite (NO assay)]. Furthermore, it reduced in vivo MSU-induced interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α), and IL-6 production, and increased Transforming growth factor-β (TGF-β) production. Importantly, trans-Chalcone reduced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and thereby the mRNA expression of the inflammasome components Nlrp3 (cryopyrin), Asc (apoptosis-associated speck-like protein containing a CARD), Pro-caspase-1 and Pro-IL-1β. In vitro, trans-Chalcone reduced the MSU-induced release of IL-1β in lipopolysaccharide (LPS)-primed macrophages. Therefore, the pharmacological effects of trans-Chalcone indicate its therapeutic potential as an analgesic and anti-inflammatory flavonoid for the treatment of gout.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome.
- Record: found
- Abstract: found
- Article: not found