- Record: found
- Abstract: found
- Article: found
Neuropharmacology of New Psychoactive Substances (NPS): Focus on the Rewarding and Reinforcing Properties of Cannabimimetics and Amphetamine-Like Stimulants
Read this article at
Abstract
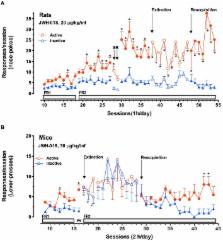
New psychoactive substances (NPS) are a heterogeneous and rapidly evolving class of molecules available on the global illicit drug market (e.g smart shops, internet, “dark net”) as a substitute for controlled substances. The use of NPS, mainly consumed along with other drugs of abuse and/or alcohol, has resulted in a significantly growing number of mortality and emergency admissions for overdoses, as reported by several poison centers from all over the world. The fact that the number of NPS have more than doubled over the last 10 years, is a critical challenge to governments, the scientific community, and civil society [EMCDDA (European Drug Report), 2014; UNODC, 2014b; Trends and developments]. The chemical structure (phenethylamines, piperazines, cathinones, tryptamines, synthetic cannabinoids) of NPS and their pharmacological and clinical effects (hallucinogenic, anesthetic, dissociative, depressant) help classify them into different categories. In the recent past, 50% of newly identified NPS have been classified as synthetic cannabinoids followed by new phenethylamines (17%) (UNODC, 2014b). Besides peripheral toxicological effects, many NPS seem to have addictive properties. Behavioral, neurochemical, and electrophysiological evidence can help in detecting them. This manuscript will review existing literature about the addictive and rewarding properties of the most popular NPS classes: cannabimimetics (JWH, HU, CP series) and amphetamine-like stimulants (amphetamine, methamphetamine, methcathinone, and MDMA analogs). Moreover, the review will include recent data from our lab which links JWH-018, a CB1 and CB2 agonist more potent than Δ 9-THC, to other cannabinoids with known abuse potential, and to other classes of abused drugs that increase dopamine signaling in the Nucleus Accumbens (NAc) shell. Thus the neurochemical mechanisms that produce the rewarding properties of JWH-018, which most likely contributes to the greater incidence of dependence associated with “Spice” use, will be described (De Luca et al., 2015a). Considering the growing evidence of a widespread use of NPS, this review will be useful to understand the new trends in the field of drug reward and drug addiction by revealing the rewarding properties of NPS, and will be helpful to gather reliable data regarding the abuse potential of these compounds.
Related collections
Most cited references185
- Record: found
- Abstract: found
- Article: not found
Neurocircuitry of addiction.
- Record: found
- Abstract: found
- Article: not found
Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study.
- Record: found
- Abstract: found
- Article: not found