- Record: found
- Abstract: found
- Article: found
A Radiometric Study of Factors Affecting Drug Output of Jet Nebulizers
Read this article at
Abstract
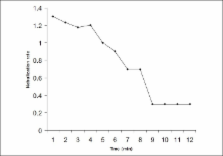
Jet nebulizers show an unreasonable variation in drug output and nebulization rates that leads to clinical and regulatory problems. Current evaluation methods appear inadequate for the purpose. Our objective was to evaluate Technetium-99m radiometry to study nebulizer parameters and the factors influencing it quantitatively. Drug output, output rate and residual mass and the effect of excipient, temperature, surface tension, air-jet speed, and equipment brand and aging were studied. Though nebulization of radiolabeled drugs followed first-order kinetics, the rates were significantly different; the heaviest drug (Tc-99m colloid) and Tc-99m salbutamol had the least nebulization. Nebulization rate for the first minute was invariably higher than the mean rate signifying the concentration effect of the solute. Drug residue was 35-75%. Drug output of different nebulizer chamber and air compressor brands was different to the extent of 270% and 180% respectively. ‘Aging’ of fluid chamber, cold drug fluid and obstruction in air-jet resulted in significant reduction in output, while addition of 2% saline as excipient did not change the output rate. Addition of ethyl alcohol resulted in a maximum of 260% enhancement (with Tc-99m salbutamol), while further reduction in surface tension was counterproductive irrespective of the drug used. We conclude that radiometry can provide valuable parametric information on the performance of different jet nebulizers.
Related collections
Most cited references52
- Record: found
- Abstract: not found
- Article: not found
The science of nebulised drug delivery.
- Record: found
- Abstract: found
- Article: not found
Targeted drug-aerosol delivery in the human respiratory system.
- Record: found
- Abstract: found
- Article: not found