- Record: found
- Abstract: found
- Article: found
CD44 Plays a Functional Role in Helicobacter pylori -induced Epithelial Cell Proliferation
Read this article at
Abstract
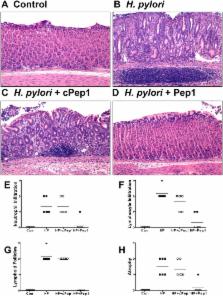
The cytotoxin-associated gene (Cag) pathogenicity island is a strain-specific constituent of Helicobacter pylori ( H. pylori) that augments cancer risk. CagA translocates into the cytoplasm where it stimulates cell signaling through the interaction with tyrosine kinase c-Met receptor, leading cellular proliferation. Identified as a potential gastric stem cell marker, cluster-of-differentiation (CD) CD44 also acts as a co-receptor for c-Met, but whether it plays a functional role in H. pylori-induced epithelial proliferation is unknown. We tested the hypothesis that CD44 plays a functional role in H. pylori-induced epithelial cell proliferation. To assay changes in gastric epithelial cell proliferation in relation to the direct interaction with H. pylori, human- and mouse-derived gastric organoids were infected with the G27 H. pylori strain or a mutant G27 strain bearing cagA deletion (∆ CagA::cat). Epithelial proliferation was quantified by EdU immunostaining. Phosphorylation of c-Met was analyzed by immunoprecipitation followed by Western blot analysis for expression of CD44 and CagA. H. pylori infection of both mouse- and human-derived gastric organoids induced epithelial proliferation that correlated with c-Met phosphorylation. CagA and CD44 co-immunoprecipitated with phosphorylated c-Met. The formation of this complex did not occur in organoids infected with ∆ CagA::cat. Epithelial proliferation in response to H. pylori infection was lost in infected organoids derived from CD44-deficient mouse stomachs. Human-derived fundic gastric organoids exhibited an induction in proliferation when infected with H. pylorithat was not seen in organoids pre-treated with a peptide inhibitor specific to CD44. In the well-established Mongolian gerbil model of gastric cancer, animals treated with CD44 peptide inhibitor Pep1, resulted in the inhibition of H. pylori -induced proliferation and associated atrophic gastritis. The current study reports a unique approach to study H. pylori interaction with the human gastric epithelium. Here, we show that CD44 plays a functional role in H. pylori-induced epithelial cell proliferation.
Author Summary
Chronic gastric inflammation, typically caused by Helicobacter pylori ( H. pylori), is the most consistent lesion leading to cancer. During a well-choreographed interaction between H. pylori and the host, the progression from chronic inflammation to cancer involves gastric epithelial changes with evidence of hyperproliferation. Our knowledge of H. pylori pathogenesis is predominantly based on data generated from gastric cancer cell lines or animal models of inflammation. We report the development and use of a novel model of primary human and mouse cultured gastric epithelial cells that are organized into three-dimensional spheroid units containing a lumen, known as gastric organoids. To assay changes in gastric epithelial cell proliferation in relation to the direct interaction with H. pylori, human- and mouse-derived gastric organoids were infected with the bacteria. Cluster-of-differentiation gene (CD44) is a transmembrane receptor responsible for epithelial cell proliferation. We show that CD44 plays a functional role in H. pylori-induced proliferation. In a Mongolian gerbil animal model of H. pylori-induced gastric cancer, we show that inhibiting CD44 blocks epithelial proliferation and subsequently cancer progression in response to bacterial infection. Thus our study provides new insights into the role of CD44 in H. pylori-induced hyperproliferation and progression of gastric disease.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994.
- Record: found
- Abstract: found
- Article: not found
Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration.
- Record: found
- Abstract: found
- Article: not found