- Record: found
- Abstract: found
- Article: found
Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets
Read this article at
Abstract
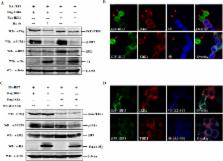
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel and highly pathogenic human coronavirus and has quickly spread to other countries in the Middle East, Europe, North Africa and Asia since 2012. Previous studies have shown that MERS-CoV ORF4b antagonizes the early antiviral alpha/beta interferon (IFN-α/β) response, which may significantly contribute to MERS-CoV pathogenesis; however, the underlying mechanism is poorly understood. Here, we found that ORF4b in the cytoplasm could specifically bind to TANK binding kinase 1 (TBK1) and IκB kinase epsilon (IKKε), suppress the molecular interaction between mitochondrial antiviral signaling protein (MAVS) and IKKε, and inhibit IFN regulatory factor 3 (IRF3) phosphorylation and subsequent IFN-β production. Further analysis showed that ORF4b could also inhibit IRF3 and IRF7-induced production of IFN-β, whereas deletion of the nuclear localization signal of ORF4b abrogated its ability to inhibit IRF3 and IRF7-induced production of IFN-β, but not IFN-β production induced by RIG-I, MDA5, MAVS, IKKε, and TBK-1, suggesting that ORF4b could inhibit the induction of IFN-β in both the cytoplasm and nucleus. Collectively, these results indicate that MERS-CoV ORF4b inhibits the induction of type I IFN through a direct interaction with IKKε/TBK1 in the cytoplasm, and also in the nucleus with unknown mechanism. Viruses have evolved multiple strategies to evade or thwart a host’s antiviral responses. A novel human coronavirus (HCoV), Middle East respiratory syndrome coronavirus (MERS-CoV), is distinguished from other coronaviruses by its high pathogenicity and mortality. However, virulence determinants that distinguish MERS-CoV from other HCoVs have yet to be identified. MERS-CoV ORF4b antagonizes the early antiviral response, which may contribute to MERS-CoV pathogenesis. Here, we report the identification of the interferon (IFN) antagonism mechanism of MERS-CoV ORF4b. MERS-CoV ORF4b inhibits the production of type I IFN through a direct interaction with IKKε/TBK1 in the cytoplasm, and also in the nucleus with unknown mechanism. These findings provide a rationale for the novel pathogenesis of MERS-CoV as well as a basis for developing a candidate therapeutic against this virus.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia.
- Record: found
- Abstract: found
- Article: not found
Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome
- Record: found
- Abstract: found
- Article: not found