- Record: found
- Abstract: found
- Article: found
Blood Eosinophil Count and Hospital Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Abstract
Purpose
This retrospective, observational cohort study investigated the association of blood eosinophil counts within 1 week of hospitalization for acute exacerbation of COPD (AECOPD) with subsequent risk of all-cause and COPD-related readmission from a large integrated health system.
Patients and Methods
Electronic medical records were extracted for index hospitalization for AECOPD at all Intermountain Healthcare hospitals. The primary outcome was the relationship of blood eosinophil count to 30-day all-cause readmission; secondary outcomes were 60-day, 90-day, and 12-month all-cause readmission, COPD-related readmission, and empiric derivation of the eosinophil count with the highest area under the curve (AUC) for predicting 30-day all-cause readmission.
Results
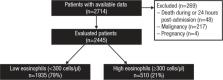
Of 2445 included patients, 1935 (79%) had a blood eosinophil count <300 cells/µL and 510 (21%) had a count ≥300 cells/µL. Using a 300-cells/μL threshold, there was no significant difference between high and low eosinophil groups in 30-day (odds ratio [OR]=1.05, 95% confidence interval [CI]=0.75–1.47) or 60-day (OR=1.15, 95% CI=0.88–1.51) all-cause readmissions. However, patients with greater (versus lesser) eosinophil counts had increased 90-day and 12-month all-cause readmissions (OR=1.35, 95% CI=1.06–1.72, and OR=1.32, 95% CI=1.07–1.62). COPD-related readmission rates were significantly greater for patients with greater (versus lesser) eosinophil counts at 30, 60, and 90 days and 12 months (OR range=1.52–1.97). A total of 70 cells/µL had the most discriminatory power to predict 30-day all-cause readmission (highest AUC).
Conclusion
Eosinophil counts in patients with COPD were not associated with a difference in 30-day all-cause readmissions. However, greater eosinophil counts were associated with increased risk of all-cause readmission at 90 days and 12 months and COPD-related readmission at 30, 60, and 90 days and 12 months. Patients with eosinophils <70 cells/μL had the lowest risk for 30-day all-cause readmission. Blood eosinophils in patients hospitalized with AECOPD may be a useful biomarker for the risk of hospital readmission.
Most cited references37
- Record: found
- Abstract: found
- Article: found
Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD
- Record: found
- Abstract: found
- Article: not found
Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial
- Record: found
- Abstract: found
- Article: not found