- Record: found
- Abstract: found
- Article: found
Nocturnal eating disturbs phosphorus excretion in young subjects: a randomized crossover trial
Read this article at
Abstract
Background
Nocturnal eating have recently increased. Serum phosphorus levels and regulators of phosphorus have circadian variations, so it is suggested that the timing of eating may be important in controlling serum phosphorus levels. However, there have been no reports on the effects of nocturnal eating on phosphorus metabolism.
The objective was to evaluate the effects of nocturnal eating on phosphorus metabolism.
Methods
Fourteen healthy men participated in two experimental protocols with differing dinner times. The design of this study was a crossover study. The subjects were served test meals three times (breakfast; 07:30 h, lunch; 12:30 h, dinner; 17:30 or 22:30 h) a day. Blood and urine samples were collected to assess diurnal variation until the following morning.
Results
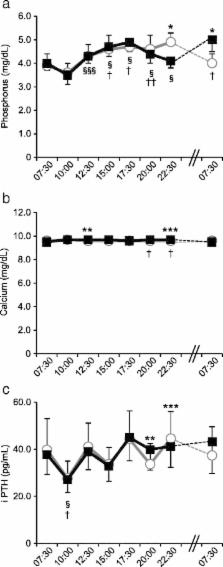
The following morning, fasting serum phosphorus levels in the late dinner group were markedly higher than those in the early dinner group ( p < 0.001), although serum calcium levels were maintained at approximately constant levels throughout the day in both groups. Fluctuations in urinary calcium excretion were synchronized with the timing of dinner eating, however, fluctuations in urinary phosphorus excretion were not synchronized. Urinary phosphorus excretions at night were inhibited in the late dinner group. In the late dinner group, intact parathyroid hormone levels didn’t decrease, and they were significantly higher in this group compared with the early dinner group at 20:00 h ( p = 0.004). The following morning, fasting serum fibroblast growth factor 23 levels in the late dinner group had not changed, but those in the early dinner group were significantly increased ( p = 0.003). Serum free fatty acid levels before dinner were significantly higher in the late dinner group compared with the early dinner group.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Relation between serum phosphate level and cardiovascular event rate in people with coronary disease.
- Record: found
- Abstract: not found
- Article: not found
Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism
- Record: found
- Abstract: found
- Article: not found