- Record: found
- Abstract: found
- Article: not found
Greater Dose-Ranging Effects on A1C Levels Than on Glucosuria With LX4211, a Dual Inhibitor of SGLT1 and SGLT2, in Patients With Type 2 Diabetes on Metformin Monotherapy
Read this article at
Abstract
OBJECTIVE
To assess the dose-ranging efficacy and safety of LX4211, a dual inhibitor of sodium–glucose cotransporter (SGLT) 1 and SGLT2, in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Type 2 diabetic patients inadequately controlled on metformin were randomly assigned to 75 mg once daily, 200 mg once daily, 200 mg twice daily, or 400 mg once daily of LX4211 or placebo. Primary end point was A1C change from baseline to week 12. Secondary end points included changes in blood pressure (BP) and body weight.
RESULTS
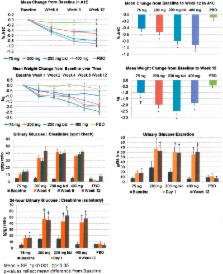
Baseline characteristics in 299 patients randomly assigned to LX4211 or placebo in this 12-week dose-ranging study were similar: mean age 55.9 years, A1C 8.1% (65 mmol/mol), BMI 33.1 kg/m 2, and BP 124/79 mmHg. LX4211 significantly reduced A1C to week 12 in a dose-dependent manner by 0.42% (4.6 mmol/mol), 0.52% (5.7 mmol/mol), 0.80% (8.7 mmol/mol), and 0.92% (10.0 mmol/mol), respectively ( P < 0.001 each), compared with 0.09% (1.0 mmol/mol) for placebo. Greater A1C reductions were produced by 400 mg once a day than 200 mg once a day LX4211 without higher urinary glucose excretion, suggesting a contribution of SGLT1 inhibition. Significant reductions were seen in body weight (−1.85 kg; P < 0.001) and systolic BP (−5.7 mmHg; P < 0.001), but diastolic BP was unchanged (−1.6; P = 0.164). Adverse events with LX4211 were mild to moderate and similar to placebo, including urinary tract infections and gastrointestinal-related events; genital infections were limited to LX4211 groups (0–5.0%). No hypoglycemia occurred.
Related collections
Most cited references19

- Record: found
- Abstract: found
- Article: found
Sodium-Glucose Cotransport Inhibition With Dapagliflozin in Type 2 Diabetes
- Record: found
- Abstract: found
- Article: not found
SGLT2 inhibition--a novel strategy for diabetes treatment.

- Record: found
- Abstract: found
- Article: found
