- Record: found
- Abstract: found
- Article: found
Perspective: Does personalized medicine hold the future for medicine?
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
With the upsurge of personalizing virtually anything such as mugs, stationery, T-shirts,
phone cases, gift items and most recently the ongoing personalized Coca-Cola UK summer
campaign, it is not surprising that medicine is fully taking root in this domain.[1]
Over the years, there has been a gradual paradigm shift from traditional medicine
as a result of increase in scientific knowledge. The traditional path of drug development
which has conventionally influenced the practice of medicine has been based on identifying
therapies which target an entire population.[2] However, there has been the recognition
of patients bearing distinctive inherent traits which cause variations in response
to therapy and subsequently tailoring medicines toward these unique responses. It
is in this light that personalized medicine (PM) evolved which is the tailoring of
treatment to the unique molecular or genetic mapping of individual patients and how
these unique features contribute to the occurrence of certain disease pattern and
progression.[3]
The concepts of PM have been subtly appreciated in medicine since the 1960s with its
fist mention in a monograph title in 1998 and subsequent publication on the Medline
interface in 1999.[4] Advancement in genetic technologies, primarily; single nucleotide
polymorphism (SNP) genotyping and microarray/biochips has been the pillar and drive
toward PM.[4] Before 1990, the issue of biomarkers was addressed in about one out
of twenty clinical trials performed. Nevertheless, since 2005, a record of 20% clinical
trials performed addressed biomarkers. Biomarkers are becoming an additional focus
area in PM as researchers are rapidly expanding knowledge in that regard.[2] The rest
of this work will initially discuss the scope of PM in present day medicine, the key
players in the development of PM and finally the future of PM.
Scope of Personalized Medicine in Present day Medicine
Conventional medicine originated from empirical treatments and gradually evolved to
mechanism-based treatments.[4] Further improvements in conventional medicine led to
the current guideline or evidence-based medicine where the approach to solving a clinical
problem is based on a number of randomized controlled trials (RCT) recognized to be
of highest level of evidence. RCT, which usually includes patients with predefined
characteristics with findings applicable to a heterogeneous patient population still
leaves room for a more tailored approach to meet the needs of peculiar cases.[5] In
this regard, some of the shortcomings in conventional medicine which PM seeks to address
include differences in treatment response and incidence of adverse reactions based
on genetic variations, exploring therapies resulting in absolute lack of efficacy
and taking into account individual variations to drug response other than applying
statistically significant outcome of an investigation involving a general population
to an individual.[4]
The advancement in Genetic Medicine forms the basis of PM. The human genome sequencing
was completed in 2000[4] and by 2003 the Human Genome Project was completed by the
Unites States department of Energy and the National Institutes of Health by identifying
22,000–23,000 genes in the human genome.[6] The human genome is the total genetic
makeup which is composed of about 3 billion nucleotides.[7] The distinct nature of
the genome to each individual provides useful information about disease development
and progression as well as response to treatment. Variations in the human genome may
be as a result of SNP, insertions and deletions, structural variants, and copy number
variation of the human genome.[4] PM is distinct from genetic medicine in that, with
PM, more complex diseases such as cancer, diabetes, neurodegenerative, and cardiovascular
diseases are addressed by taking into account both genetic and environmental factors.
Genetic medicine is limited to the clinical effect of a single genetic variation such
as in the case of dystrophy, cystic fibrosis, and sickle cell anemia.[6] The complex
nature of drug response is as a result of a combination of both genetic and environmental
factors. Predicted treatment success based on genotype test may result in poor response
due to environmental influence.[4] For example, in the management of Hepatitis C,
which is based on genetic response, nongenetic factors such as age, obesity, and alcohol
consumption have been reported to influence treatment outcomes.[8]
Another area of advancement which greatly impacts PM is Systems Biology and Pharmacology.
System Biology translates to PM by elucidating mechanism-based disease development,
disease risk estimation, preventive medicine, personalized disease, and treatment
monitoring.[9] Systems biology is most useful in preventive PM by developing tools
to detect minute changes in molecular profiles at the early stages of disease development.
It also takes into account physiological environmental factors exposed to gene expression
such as body cells, tissues, body fluids, and body surface area.[9] With regards to
Systems Pharmacology, integration into PM seeks to identity the genes and proteins
responsible for drug treatment and resistance which may be targeted to augment treatment
outcomes through synergistic effect.[10] Biomarkers are also gaining popularity in
PM. They are characteristics that measure indicators of the actual biology, disease
or drug response. Biomarkers exist as proteins, deoxyribonucleic acid (DNA), messenger
ribonucleic acid or radiological parameters which are applicable in areas of disease
risk estimation, diagnostic screening, diagnosis, prognosis, prediction, and response
monitoring.[11] One application of biomarkers is the use in determining dose size
for clinical trials. Pharmacodynamics tests are usually based on the occurrence of
toxicity to determine the maximum tolerated dose. With the application of biomarkers,
maximum doses are determined by the presence of specific biomarkers. For example,
the reduction of Ki67 in tumor tissue as a response to MEK inhibitors in cancer chemotherapy
can be used to ascertain appropriate dose size based on the extent of reduction of
the Ki67 biomarker.[11]
Moving on to drug development, PM has found its place in drug discovery, clinical
trial design and the practice of medicine. For drug discovery, one of the earliest
discoveries pertains to trastuzumab which is used in breast cancer. This was targeted
at the approximate 30% of breast cancer patients who were unresponsive to standard
treatment due to over-expression of Human Epidermal Growth Factor Receptor 2.[12]
Another discovery is in cardiovascular medicine whereby genetic based noninvasive
diagnostic test has replaced the invasive endomyocardial biopsy.[12] Further discovery
in cancer therapy is the discovery of vemurafenib; a B-Raf protein inhibitor targeted
at the human genome known as BRAF, which is responsible for the development of melanoma.[12]
In clinical trial design, PM is gradually redefining the traditional RCT and implementing
trials with no control or placebo arms. Furthermore, targeted predictive trials as
presented through PM eliminate ethical concerns of administering placebo or no treatment
to patients when standard treatments are available. Most oncology drugs are approaching
PM trial design and a typical example is the success of Crizotinib; an anaplastic
lymphoma kinase-echinoderm microtubule-associated protein-like 4 (ALK-EML4) inhibitor.
ALK-EML4 fusion is the driver for lung cancer progression. The Crizotinib trial included
82 lung cancer patients tested positive for the biomarker ALK with no control group.
Favorable results of tumor shrinkage were observed as early as after 48 h with statistically
significant outcome as well.[13]
In the practice of medicine, PM has facilitated genetic-based assessment of efficacy,
adverse drug reactions, and appropriate dose regimen. For example, abacavir is reported
to yield hypersensitivity reactions in some patients. Traditionally, these hypersensitivity
reactions were diagnosed after clinical manifestations of symptoms. A study by Mallal
et al.,[14] proved a genetic linkage with the hypersensitivity by identifying the
driving gene as the major histocompatibility complex class I allele HLA-B*57:01. Upon
investigation, patients who were tested negative for this gene were not hypersensitive
to abacavir and vice versa. This steered both the Food and Drugs Administration (FDA)
and European Medicines Agency to require of the pharmaceutical industries to include
in their label precautions the need to have gene testing prior to abacavir therapy
(Novelli, 2010 cited by Pucheril (2011)).[3]
Ethical considerations in PM primarily concern the protection of genetic information
and other private information of participating individuals. In the USA, the Genetic
Information nondisclosure Act ensures the ethical use of genetic information.[4] With
regards to PM regulation, the focus is on obtaining approval for the molecular diagnostic
tests and the drugs related to the PM. This is typical in the USA where the FDA encourages
but does not make it mandatory to submit pharmacogenetic and pharmacogenomic data
during the drug development.[4] From the economic perspective, two key concerns arise
with PM; that is, will PM render efforts by biopharmaceutical companies profitable
and can healthcare providers and patients afford PM? According to a report by PricewaterhouseCoopers,
PM is estimated to grow to US$452 billion by 2015 in USA alone.[15] Though high cost
is involved in driving PM, the wholistic benefit of PM proves cost-effective. With
improved healthcare and quality of life of patients and the targeted treatment which
eliminates wastage, PM proves to be cost-effective compared with conventional medicine.[4]
Key Players in the Development of Personalized Medicine
The development of PM is a multidisciplinary approach requiring team effort from players
which may be categorized as industrial players, the academia, scientific players,
political, and socioeconomic players.[4] The industrial players which are predominantly
the pharmaceutical industry and the biotechnology companies have taken lead roles
in the advancement of PM. Most often, the pharmaceutical companies rely on technologies
and data from biotechnology companies for the application of pharmacogenetics and
pharmacogenomics in clinical trials. However, among the top five pharmaceutical companies
(i.e. Hoffman-La Roche, GlaxoSmithKline, AstraZeneca, Perlegen Sciences Inc., and
Clinical Data Inc.,) advancing in PM, Hoffman-La Roche has strategically distinguished
and positioned itself to be at the top of the ladder by pioneering in the development
of products which integrates both diagnostics and therapeutics.[4] An example of such
product by Hoffman-La Roche is the drug vemurafenib and its diagnostic tool BRAF V600E
Mutation Test for the management of melanoma.[3] Likewise, smaller biotechnology companies
after innovating technologies do rely on the pharmaceutical companies or other big
biotechnology companies for production.
Both the pharmaceutical industry and biotechnology companies also collaborate with
academic institutions in the development of PM. One such coalition is the PM Coalition
located in Washington DC. It is a nonprofit organization, whose membership is open
to interest groups in PM such as pharmaceutical industry, biotechnology companies,
universities, government agencies, patient groups, information technology companies,
and healthcare providers.[16] In facilitating the coalition with academic institutions,
the industrial partners make available patient data from clinical trials to the academic
researchers for synthesis of results and further research. The results obtained by
the academic researchers are relayed to the industry partners for input into bioinformatics
tools developed by the industry. Examples of such collaborations include Pfizer/Harvard
Medical School, Perlegen/George Washington University and Pathway Diagnostics/Duke
University.[4]
The scientific players include clinical laboratories, Health Information Technology
(HIT) and medical professionals. Clinical laboratories usually fall within the biomedical
sciences which carry out most of the genetic projects. In the USA, Genomas®; a biomedical
company with a specialized unit into PM known as Laboratory of Personalized Health
received a license to expand in New York, Florida, and California. By 2012, the PM
services of Genomas® had extended to Texas, Pennslyvania, and Connecticut. In Connecticut,
a record of 500 clinicians and 5000 patients had benefited from LHP covering disease
such as diabetes, heart diseases, and neuropsychiatric drugs.[17] HIT is also contributing
hugely to the advancement of PM. Such technology creates a central electronic system
which enables the smooth flow of information such as medical images, genomics, biospecimens,
and patient outcomes among collaborating partners whiles ensuring protection of data.
This system of communication is effectively being applied through a system known as
the cancer Biomedical Informatics Grid, launched in 2003 by the National Cancer Institute
(NCI), USA. Through this network, more than 50 NCI cancer units together with academic
and commercial institutions share biomedical information in a more integrated way
with an open source software applications.[18] Medical professions are also key scientific
drivers as they are at the application end of the entire genomic findings. Efforts
are being made to expand the knowledge of medical professionals in genomics through
continuing education programs organized at conferences and symposia sponsored by the
biopharmaceutical industries.[4]
Last but not least, political and socioeconomic players drive medicine toward PM.
Especially in the USA and Europe, policies have been made to integrate PM into healthcare
systems as well as funding toward genomic projects.[4] Health Insurance companies
too on the other end are supporting the shift toward PM in order to minimize expenditure
on ineffective treatments and long duration of trial-and-error treatments for clients.[4]
The general public with its enormous pressure on the government and pharmaceutical
industries to provide safer and more effective treatments have also supported activities
toward PM. A typical example is the Personalized Genome Project which started with
only 10 volunteers is currently recording 2086 volunteers.[19]
Future of Personalized Medicine
The prospect of PM being an integral part of detecting, managing and preventing diseases
is primarily dependent on its progress and impact of PM in field medicine. Progress
has been seen in areas such as ongoing genomic projects, merging translational medicine
with PM, increasing advance toward personal genetic testing, and the observed evolution
of conventional medicine to PM.
Many ongoing genomic projects are targeted at building a strong foundation toward
PM. For example, much progress has been made in the molecular diagnosis of breast
cancer based on molecules such as hormone receptors and ribonucleic acid.[20] However,
there is little application of these molecular diagnostic tools to understand the
genetic basis of disease occurrence and progression.[4] In the same subject area of
breast cancer, the Cancer Research UK has funded the first and largest ever genetic
research into breast cancer in 2005. This high-resolution, whole genome association
study on breast cancer involves the collaborative efforts of Cancer Research UK, the
University of Cambridge, Cancer Research Technology and Perlegen Sciences, Inc. The
aim of this project was to determine over 200 million individual genotypes in the
DNA samples of patients in order to understand the genetic basis of the disease in
the area of prevention, early detection, and treatment.[21] In the same year; 2005,
a 15-year research project has also been funded by the National Institute of Health
to research into the understanding of the genetic basis of coronary heart disease,
stroke, and breast cancer in relation to postmenopausal hormone therapy in 161,808
women between the ages of 50 and 79. This is also a collaboration between Perlegen
Sciences, Inc., and Women's Health Initiative based on high density whole genome scan
of SNPs.[22] These efforts toward the genetic understanding of diseases are paving
the way toward PM.
Another ongoing genomic project is the Personal Genome Project initiated by George
M. Church of Harvard University in 2005 with the aim of making personal genomes available
to the general public and foster rapid dialog with interest groups at a low cost.
The target of this project is to enroll 100,000 volunteers and have currently achieved
2806 volunteers having started with only 10 volunteers including George Church. The
gathered genetic information is aimed at individualizing disease risk factors, biological
characteristics, and personal ancestries.[19] Furthermore, the increasing approach
toward Genome-Wide Association Studies (GWAS) since the completion of the human genome
project has successfully found genetic based variations in risk factors for diseases
such as type 2 diabetes, heart disorders, Pakinson's disease, obesity, prostate cancer,
and Crohn's disease. The main goal of the GWAS is to hasten the scanning of markers
across genomes of many patients to identify genetic variations associated with particular
diseases which will enable more tailored detection, prevention and treatment of diseases.[7]
In addition, the 1000 Genomes Project which is the first project to undertake genomic
sequencing in a large population since its inception in 2008 seeks to identify genetic
variants occurring in at least 1% of the population.[23]
Moreover, another area of advancement which is pointing toward PM is in the field
of Translational Science (TS). TS is concerned with the transfer of preclinical technologies
to clinical application.[4] In recent advancement, the methods applied in TS are more
inclined to PM. These applications include the use of biomarkers to predict potency
as well as assess toxicity, developing animal models which mimic human disease pattern,
bioinformatics, and creating a similar image analysis software for both preclinical
and clinical studies in order to reduce failure rates at later stages of drug development.[4]
Again, a further progress toward PM is the collaborative study of Scripps Translational
Institute, Navigenics, Affymetrix, and Microsoft to investigate the long-term effects
of personal genetic testing.[24] This study hopes to ascertain whether personal genetic
testing contributes to an individual decision in making healthy lifestyle choices
such as exercising and proper diet habits. The overall aim is to provide individualized
guidelines toward decreasing health risks based on personal genetic information.[24]
With all these leveling out on the drawing board, conventional medicine is being evolutioned
into PM without a doubt. The increasing genomic knowledge coupled with newer technologies
such as bioinformatics is inevitably retiring medical professionals trained in the
prebiotechnology era to give way for those abreast with the knowledge in the areas
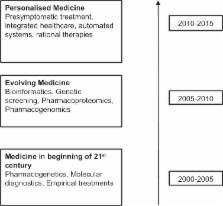
such as molecular medicine, pharmacogenomics and pharmacogenetics [Figure 1]. Again,
there is a mounting pressure on government agencies from the public to provide safer
and more effective medications coupled with political pressure to reduce health bills
through the delivery of effective treatments whiles minimizes expenditure on ineffective
therapies.[4] An example of such move is the passing of the Genomics and Personalized
Medicine Act of 2006[25] in the USA in its attempts to bridge the gap between conventional
medicine and PM.
Figure 1
Evolution of personalized medicine as a market driver[4]
All these efforts toward impacting PM in today's medicine and the future offers benefits
particularly to patients, physicians, biopharmaceutical industries, and the society
at large. The impact of PM on patients is significant. Primarily, PM offers patient
treatments with high precision of being effective saving them time in trial-and-error
with less effective treatments. Avoiding trial-and-error treatments also lowers the
cost of treatment and minimizes the risk of unwanted adverse reactions.[3] For instance,
in conventional medicine, cancer chemotherapy will be administered to a patient on
the basis of the treatment having statistically significant clinical outcome in a
trial population. However, a fraction of patients not responding to the treatment
will have to suffer the associated adverse effects, bear the pain of disease progress
as well as incur loss to the healthcare provider or themselves. One such breakthrough
is seen with metastatic colorectal cancer where newer chemotherapies such as panitumumab
and cetuximab have been developed to target patients identified with KRAS gene who
show less improvement with the three conventional chemotherapies namely: 5-fluorouracil,
irinotecan, and oxaliplatin.[26] Above all, PM contributes to an improved quality
of life for both patients who get effective treatment as well as in healthy individuals
through personalized preventive healthcare. The results of patient satisfaction from
PM is directly related to the satisfactory outcome physicians obtain from the implementation
of PM. PM enables physicians to avoid trial-and-error approach to diagnosis and treatment
based on the molecular and genetic basis of disease development.
Another benefactor of PM is the biopharmaceutical industry. The fundamental principle
of drug development is to minimize cost and time of development. The process of trial-and-error
is very time consuming as the outcome cannot be predicted and alarming outcomes from
a single individual who is genetically intolerant to the test drug can bring the entire
drug development process to a halt. A similar situation is reported of the death of
Ellen Roche who participated in an Asthma clinical trial to observe the “Mechanisms
of Deep Inspiration-Induced Airway Relaxation.” Two other healthy volunteers who received
the same dose of hexamethonium reported dry cough in one patient which was later resolved
while the other experienced no ill health. Perhaps, if predictive modeling based on
genetic response was applied, these variations in response may have been alerted to
avoid the calling off of the entire research. In addition, nearly all federal funded
trials at John Hopkins were also suspended as a result of the incident.[27]
Still on the biopharmaceutical industry, PM enhances discovery of new safer and more
effective treatments which can also drive monopoly in the market. About 20% of genomes
is patented rendering huge revenue to the owners.[28] One other amazing advantage
PM is the hope rescue of failed drugs whereby a drug which may present unacceptable
efficacy and toxicity in the larger population may prove favorable in a peculiar set
of patients. Two drugs for example, have been salvaged through the application of
PM; namely Thalidomide and Clozapine. Despite Thalidomide's stigma following fetal
deformities in pregnant woman, its usefulness has been revived as a result of proven
efficacy and safety in multiple myeloma and Crohn's disease.[29] Clozapine which has
also reported life-threatening adverse effects of agranulocytosis has survived the
market due to its safety and efficacy in some patient population with schizophrenia.
Current investigations of clozapine are targeted at depression, Parkinson's disease,
and Huntington's chorea.[30]
In the midst of all the above mentioned positive prospects of PM coupled with its
presenting advantages, certain limitations do pose a threat to the rising success
story. First and foremost, not all treatments can be personalized. A typical challenge
PM faces in this domain is the personalization of treatment for common diseases.[31]
Common diseases have wide genetic variants which are rarely studied as much emphasis
is being placed on complicated ailments such as cancer and metabolic disorders. Another
challenge facing the personalization of common diseases is the fact that identification
of rare genes will end up resulting in millions of rare genes because of the large
population size and tailored treatment will mean developing thousands of treatments
for the same condition.[31] This obviously will limit the extension of PM toward common
diseases such as common cold, malaria and diarrhea.
A second limitation of PM is the fact that other external factors other than genes
contribute to drug response. Diet, lifestyle, and infections do influence the genetic
response to drugs. This implies that, people may have certain genetic variant but
unless they are exposed to a particular disease, that variant becomes irrelevant.
Vice versa, diet or lifestyle of an individual may alter the response of a genetic
variant an individual may possess to targeted treatment which may complicate the success
of PM.[31] A third limitation is the limited support from government and other healthcare
organizations. Ideally, PM should be advanced across the globe since genetic variants
are manifested in the broad ethnic domain. In the developed countries such as USA
and Europe, PM have been acknowledged and implemented in health policies to foster
its development. Third world countries which are lagging behind even in conventional
medicine will obviously have limited resource toward PM. For example, the current
available data on pharmacogenetics does not give comprehensive information with regards
to variations in drug response across all human population because the data entries
are solely from the white race.[4] With regards to the healthcare organizations, human
resource is lacking in genetic science areas such as bioinformatics as well the implementation
of scientific tools for data management.[4]
Another challenge facing PM is ethical, legal, and social concerns. Issues have been
raised concerning stratifying patients into ethnic groups will result in social segregation
which policy makers strive to avoid. Furthermore, denying patients treatment based
on genetic classification may be poorly understood by many in the general public and
be thought of as treatment denial.[4] Within the regulatory, regulatory bodies also
pose difficult measures for obtaining approval on new biomarkers. An example is the
launch of Varisante; a biomarker-based diagnostic tool for sin cancer which approval
in Canada and Europe but approval in the USA was anticipated to delay for at least
a year. The chief executive office remarking the possibility of encountering approval
difficulty in the USA due to the FDA's recent track record of rejecting applications
on medical devices.[32] Moreover, the situation known as “incidentalome;” whereby
genetic screening results in nonrelevant data poses a threat to the advancement of
PM. This usually incurs huge costs, undue stress to patients having to undertake series
of tests and also a huge burden on researchers to handle unexpected genetic data.[4]
Summary and Conclusion
In the fast advancing era of Genomic and Molecular medicine, stakeholders are inevitably
inclining to specificity in the practice of medicine. Patient satisfaction on disease
management is centered on the demand for drug therapies to be more effective with
reduced incident of adverse effects to ensure improved quality of life. Physicians
are also welcoming therapies which will result in definite cure and minimize trial-and-error
diagnosis and treatment. In addition, medical practice is accepting the molecular
and genetic basis of assessing disease risk factors and preventive mechanisms. Pharmaceutical
and biotechnology companies are also advancing in drug development pathways, which
are quicker with much predictive outcomes in order to save time and money. Regulatory
authorities are also being pressured to approve drug therapies with minimum adverse
reactions and increase efficacy. Government agencies and healthcare agencies have
also developed an interest in more precise treatments in order to prevent expenditure
on ineffective dugs which will lengthen patients’ morbidity span and incur more health
bills. In conclusion, although conventional medicine cannot be totally ruled out,
it is evident that PM is shaping the future of medicine and stands a promising chance
of overtaking conventional medicine in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Thalidomide.
G MacPherson, William Figg, Ashley Franks (2004)
- Record: found
- Abstract: found
- Article: not found
Targeted therapy in metastatic colorectal cancer -- an example of personalised medicine in action.
- Record: found
- Abstract: found
- Article: not found
Systems biology: personalized medicine for the future?
Rui Hong Chen, Michael Snyder (2012)