- Record: found
- Abstract: found
- Article: not found
Early short-course corticosteroids and furosemide combination to treat non-critically ill COVID-19 patients: An observational cohort study
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Dear Editor,
Acute respiratory distress syndrome (ARDS), a life-threatening complication of coronavirus
disease-2019 (COVID-19) is associated with elevated risk of intensive care unit (ICU)
admission and death, predominantly in the elderlies.
1
Based on a randomized controlled trial, early dexamethasone was shown effective to
reduce mechanical ventilation (MV) duration and overall mortality in moderate-to-severe
ARDS patients independently of the etiology.
2
Therefore, since systemic and pulmonary inflammatory cytokine storm and fibrinous
and organizing pneumonia are involved in COVID-19 ARDS, early corticosteroid administration
has been considered as appropriate to avoid clinical deterioration and need for MV
support.
3
However, cautious has been advised due to potential harmful effects of corticosteroids
in viral pneumonia such as COVID-19.
4
During COVID-19 epidemic, non-critically ill COVID-19 patients for whom intubation
could be an option if worsening and those not eligible for intubation due to refusal,
comorbidities and/or advanced age in the context of limited access to ICU beds were
referred to our ward. All patients received standard care, i.e. oxygen with adapted
flow to oximetry (including high-flow oxygen), antibiotics, anticoagulants, vasopressors
and antiviral drugs if needed. Usual monitoring was provided including pulse oximetry,
electrocardiogram, finger blood sugar and daily routine chemical tests. Decision to
administer corticosteroids was left to physicians in charge due to uncertainties regarding
the benefit/risk balance of their use in non-critically ill COVID-19 patients.
5
Interestingly, because pulmonary edema could worsen hypoxemia in patients presenting
cardiovascular co-morbidities and/or cardiac involvement in COVID-19 at risk of fluid
retention, we decided to co-administer furosemide systematically to corticosteroid-treated
patients, with a rationale similar to that of conservative fluid management in ARDS
patients.
5
Therefore, to address the effectiveness of early short-course corticosteroid/furosemide
treatment in the non-critically ill COVID-19 patient, we designed a retrospective
observational cohort study. All successive COVID-19 patients with pneumonia requiring
oxygen admitted to our non-critical medical ward from 03/11/2020 to 04/27/2020 were
included. Patients who received intravenous or oral corticosteroids plus furosemide
for at least once daily three consecutive days were compared to those who did not
(usual care group). The primary composite endpoint was invasive MV requirement (corresponding
to care escalation from ward to ICU) or 28-day mortality. Data are expressed as median
[percentiles 25th-75th] or percentages. Univariate comparisons were performed using
Mann-Whitney or Fisher exact tests, as appropriate. A multivariate logistic regression
model to explain the outcome was tested with the corticosteroid/furosemide treatment
as explanatory variable and adjustment for independent covariates (gender, age, body-mass
index and comorbidities). Odds ratios (OR) and their 95%-confidence intervals were
determined. P-values ≤0.05 were considered significant. Analyses were preformed using
the R3.6 environment.
One-hundred-and-nineteen patients (age, 75yrs [63-83]); M/F sex-ratio, 1.9; past hypertension,
61%; diabetes mellitus, 39%; cardiovascular diseases, 39%; lung diseases, 24%) were
included (Table 1
). Twenty-six patients received the corticosteroid/furosemide combination (prednisolone
dose equivalent, 1.25mg/kg/24h [0.85-1.87]; furosemide dose, 80mg/24h [40-100]) during
4days [3-4]) whereas ninety-three patients did not. Noteworthy, 14/24 control patients
(58%) at risk of cardiogenic pulmonary edema (serum brain natriuretic peptide (BNP)
≥100ng/mL) received furosemide without corticosteroids. In the corticosteroid/furosemide
treatment group, incidence of invasive MV or death was lower than that in the usual
care group (OR=0.35 [0.11-1.01], P=0.040). The multivariate analysis confirmed the
significant effect of the corticosteroid/furosemide treatment on outcome after adjustment
for independent covariates (OR=0.28 [0.07-0.88], P=0.038). Among covariates, male
gender (OR=5.03 [1.69-17.49], P=0.006) and maximal oxygen flow (OR=1.14 [1.01-1.32],
P=0.049) were associated with worse outcome. The model was significant compared to
a model without the corticosteroid/furosemide treatment (P=0.028). Additionally, ORs
were analyzed in patient subgroups stratified by age (using the median value as threshold),
gender and risk factors including diabetes mellitus, elevated BNP (threshold, 100ng/ml)
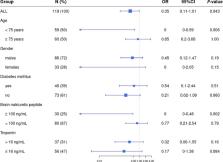
and troponin levels (threshold, 16ng/mL; Figure 1
). Remarkably, there was a significant effect of corticosteroid/furosemide treatment
in elevated-BNP patients (OR=0.00 [0.00-0.48], P=0.020) while low-BNP patients did
not appear to benefit from the treatment. Outcome was improved in elevated- versus
low-BNP patients (P=0.030; Cochran-Mantel-Haenszel test). Clinicians reported no remarkable
adverse effects attributed to the corticosteroid/furosemide treatment.
Table 1
Characteristics of the COVID-19 patients treated or not treated with the corticosteroid/furosemide
combination. Data are presented as percentages or medians [percentiles 25th-75th].
Comparisons were performed using Mann-Whitney or Fisher exact tests, as appropriate.
Table 1
Corticosteroid/furosemide-treated patients(N = 26)
Non-corticosteroid/furosemide-treated patients(N = 93)
P
Demographics and past medical history
Age (years)
75 [66-83]
76 [63-83]
0.94
Male gender, N (%)
18 (69)
68 (73)
0.80
Body mass index (kg/m²)
27 [23-33]
27 [24-29]
0.69
Past hypertension, N (%)
18 (69)
55 (59)
0.37
Diabetes mellitus, N (%)
13 (50)
33 (35)
0.25
Past cardiovascular disease, N (%)
11 (42)
36 (39)
0.82
Chronic lung disease, N (%)
7 (27)
22 (24)
0.80
Clinical and biological parameters on admission
Symptom duration (days)
8 [4-10]
9 [5-10]
0.85
SpO2 at room air (%)
92 [88-96]
92 [90-94]
0.94
PaO2 at room air (mmHg)
67 [58-78]
62 [57-75]
0.48
Maximal oxygen flow (L/min)
3.5 [1.25-5]
3.0 [2-4]
0.65
High-flow oxygen, N (%)
2 (8)
7 (8)
1.00
C-reactive protein (mg/L)
121 [35-171]
106 [55-147]
0.44
Procalcitonin (µg/L)
0.16 [0.10-0.34]
0.20 [0.07-0.46]
0.84
White blood cells (G/L)
6.5 [5.3-9.1]
6.8 [5.2-8.8]
0.99
Lymphocytes (G/L)
0.95 [0.69-1.08]
0.94 [0.65-1.22]
0.66
Brain natriuretic peptide (ng/L)
38 [12-95]
47 [16-138]
0.71
Troponin Ic high-sensitivity (ng/mL)
18 [7-28]
11 [5-27]
0.27
D-dimer (ng/mL)
1,050 [640-2,018]
1,350 [745-2,418]
0.66
Additional treatments
Antibiotics, N (%)
25 (96)
77 (82)
0.19
Prophylactic anticoagulant, N (%)
25 (96)
91 (98)
0.52
Furosemide, N (%)
26 (100)
27 (29)
<0.0001
Hydroxychloroquine, N (%)
4 (15)
16 (17)
1.00
Lopinavir/ritonavir, N (%)
2 (8)
5 (5)
0.65
Anti-interleukin-6 receptor, N (%)
1 (4)
0 (0)
0.22
Outcome
Mechanical Ventilation requirement or 28-day death, N (%)
6 (23)
43 (46)
0.04
Mechanical Ventilation, N (%)
0 (0)
10 (11)
0.12
Length of hospital stay (days)
14 [10-21]
9 [5-16]
0.007
28-day death, N (%)
6 (23)
33 (35)
0.34
Fig. 1
Impact of the corticosteroid/furosemide treatment in the different patient subgroups
defined according to age (using the median value as threshold), gender, presence of
diabetes mellitus, serum brain natriuretic peptide (BNP; threshold at 100 ng/mL) and
troponin levels (threshold at 16 ng/mL). Odds ratio (OR) and their 95%-confidence
intervals were determined.
Fig 1
Our findings are consistent with the retrospective analysis from the large Chinese
dataset reporting that methylprednisolone exposure was significantly beneficial in
COVID-19 patients admitted with ARDS
1
. The randomized controlled open-label RECOVERY trial showed that dexamethasone 6mg
given once daily for up to ten days reduced 28-day mortality by one-third among mechanically
ventilated COVID-19 patients and by one-fifth among patients treated with oxygen,
while no benefit was observed in patients not receiving respiratory support at randomization.
7
In COVID-19 patients, viral shedding is elevated early then declines. Interestingly,
low-dose corticosteroids were shown not to delay viral clearance, thus encouraging
their safe prescription aiming to limit the excessive systemic and pulmonary inflammation
involved in ventilation worsening.
8
However, the best dose regimen and timing of corticosteroids in COVID-19 remain undetermined.
Various anti-inflammatory therapies including interleukin-1-receptor and interleukin-6
receptor antagonists were proposed to treat non-critically ill COVID-19 patients.
9
However, availability and cost-effectiveness of corticosteroids/furosemide (∼60-fold
less expensive than monoclonal antibodies) remain unbeatable.
In aged COVID-19 patients with high proportion of cardiac comorbidities, mild-to-moderate
pneumonia may be accompanied by some degree of acute heart failure and ischemia,
10
as evidenced in our series by elevations in cardiac biomarkers (BNP, 43 ng/l [16-135]
and troponin, 12 ng/ml [5-27], respectively). Thus, furosemide administration when
prescribing corticosteroids is pertinent, possibly beneficial to limit corticosteroid-induced
retention and at least safe if adequately monitored. We observed increase in length
of hospital stay, undoubtedly corresponding to increased survival resulting in prolonged
medical care and rehabilitation.
Our study limitations include the non-randomized single-center design and relatively
small number of patients. The brief study duration determined by COVID-19 epidemic
duration precluded a more elaborate design. Future trials should determine the most
appropriate strategy offering the best risk/benefit ratio.
To conclude, our data provides evidence that early short-course of corticosteroids
combined to furosemide reduces the risk of invasive MV requirement or 28-day mortality
in the non-critically ill COVID-19 patients. In comparison to the RECOVERY trial results,
our findings highly suggest the benefits and safety of adding furosemide to corticosteroids,
aiming to improve fluid management especially in the aged patients with comorbidities
at risk of pulmonary edema (BNP >100ng/mL on admission).
Uncited References:
6
Declaration of Competing Interest
The authors declare that they have no competing interests.
Related collections
Most cited references7
- Record: found
- Abstract: found
- Article: found
COVID-19: consider cytokine storm syndromes and immunosuppression
Puja Mehta, Daniel McAuley, Michael Brown … (2020)
- Record: found
- Abstract: found
- Article: not found
Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China
Chaomin Wu, Xiaoyan Chen, Yanping Cai … (2020)
- Record: found
- Abstract: found
- Article: not found
Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury
Clark D. Russell, Jonathan Millar, J Baillie (2020)
