- Record: found
- Abstract: found
- Article: found
Changes in Circulating Lysyl Oxidase-Like-2 (LOXL2) Levels, HOMA, and Fibrosis after Sustained Virological Response by Direct Antiviral Therapy
Read this article at
Abstract
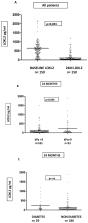
Background: we aimed to assess the influence of metabolic syndrome on fibrosis regression (using liver-stiffness measurement (LSM) and serological scores) and the relationship with the expression of lysyl oxidase-like-2 as a potential goal of antifibrotic therapy. Methods: We included 271 patients treated with Direct Antiviral Therapy (DAAs) in our hospital who achieved a sustained virological response (SVR); physical examination, blood tests, and LSM were made at baseline (B) and 24 months (24 M) after SVR. Hemodynamic studies and transjugular liver biopsies were performed on 13 patients. Results: At B, 68 patients were F1 (25.1%); F2 n = 59 (21.7%); F3 n = 44 (16.05%); and 100 were F4 (36.9%). Although the LSM (absolute value) improved in 82% of patients ( n = 222), it progressed in 17.5% of patients ( n = 48). At 24 M, 48 patients met the metabolic syndrome (MetS) criteria and there was an increase in patients with a BMI of >25 kg/m 2 ( p < 0.001). At B and 24 M, a BMI of >25 kg/m 2 is a risk factor for significant fibrosis or steatosis at 24 M ( p < 0.05) and progression on LSM ( p < 0.001), as well as MetS at B and 24 M (OR 4.1 IC (1.4–11.7), p = 0.008; and OR 5.4 IC (1.9–15.4), p = 0.001, respectively). Regarding the correlation between LSM and the liver biopsy, we found that only six out of 13 patients had a matching LSM and biopsy. We found a statistically significant decrease in LOXL2 levels at 24 M with respect to B ( p < 0.001) with higher serological value in patients with elastography of >9 kPa vs. <9 kPa ( p = 0.046). Conclusion: Regression of LSM was reached in 82% of patients. Downregulated LOXL2 was demonstrated post-SVR, with overexpression in cirrhotic patients being a potential therapy goal in selected patients.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment.
- Record: found
- Abstract: found
- Article: not found