- Record: found
- Abstract: found
- Article: not found
Targeting cellular senescence prevents age-related bone loss in mice

Abstract
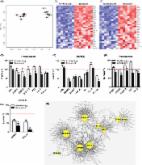
Aging is associated with increased cellular senescence, which is hypothesized to drive the eventual development of multiple co-morbidities 1 . Here, we investigate a role for senescent cells in age-related bone loss by multiple approaches. In particular, we used either genetic ( i.e., the INK-ATTAC “suicide” transgene encoding an inducible caspase 8 expressed specifically in senescent cells 2– 4 ) or pharmacological ( i.e., “senolytic” compounds 5, 6 ) means to eliminate senescent cells. We also inhibited the production of the pro-inflammatory secretome of senescent cells using a JAK inhibitor (JAKi) 3, 7 . In old (20–22-months) mice with established bone loss, activation of the INK-ATTAC caspase 8 in senescent cells or treatment with senolytics or the JAKi for 2–4 months resulted in higher bone mass and strength and better bone microarchitecture compared to vehicle-treated mice. The beneficial effects of targeting senescent cells were due to lower bone resorption with either maintained (trabecular bone) or higher (cortical bone) bone formation as compared to vehicle-treated mice. In vitro studies demonstrated that senescent cell-conditioned medium impaired osteoblast mineralization and enhanced osteoclast progenitor survival, leading to increased osteoclastogenesis. Collectively, these data establish a causal role for senescent cells in bone loss with aging and demonstrate that targeting these cells has both anti-resorptive and anabolic effects on bone. As eliminating senescent cells and/or inhibiting their pro-inflammatory secretome also improves cardiovascular function 4 , enhances insulin sensitivity 3 , and reduces frailty 7 , targeting this fundamental mechanism to prevent age-related bone loss suggests a novel treatment strategy not only for osteoporosis but also for multiple age-related co-morbidities.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments
- Record: found
- Abstract: found
- Article: found
The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs
- Record: found
- Abstract: found
- Article: not found
