- Record: found
- Abstract: found
- Article: found
Standardized Electrolyte Supplementation and Fluid Management Improves Survival During Amphotericin Therapy for Cryptococcal Meningitis in Resource-Limited Settings
Author(s): Nathan C. Bahr, Melissa Rolfes, Abdu Musubire, Henry Nabeta, Darlisha A. Williams, Joshua Rhein, Andrew Kambugu, David B. Meya, David Boulware
Publication date: 2014-08-25
Journal: Open Forum Infectious Diseases
Publisher: Oxford University Press
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
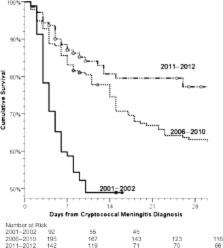
Cryptococcal meningitis is the most common cause of adult meningitis in sub-Saharan Africa [1–3], and among persons infected with human immunodeficiency virus (HIV) it accounts for 20%–25% of acquired immune deficiency syndrome-related mortality in Africa [4–7]. Standard treatment is combination therapy with amphotericin and flucytosine (or with fluconazole when flucytosine is unavailable) [8, 9]. Although amphotericin-based regimens have superior clinical efficacy over fluconazole monotherapy [10], amphotericin has side effects including nonlife-threatening infusion-related reactions (eg, rigors, fevers, chills, nausea, and vomiting) as well as more significant cumulative toxicities such as nephrotoxicity, anemia, hypokalemia, and hypomagnesemia [11–13]. In persons receiving amphotericin B deoxycholate for >10 days, hypomagnesemia and hypokalemia are near universal (96% and 100%, respectively) [14]. Yet unlike in high-income countries, potassium (K+) and magnesium (Mg2+) monitoring and electrolyte replacement are limited in low- and middle-income countries. In many settings, electrolyte monitoring is much more limited or absent, and electrolyte replacement is more sporadic and physician-dependent. Little data exist on how best to optimize management of electrolytes in patients given amphotericin in resource-limited settings. In the second month (January 2011) of the Cryptococcal Optimal Antiretroviral Therapy Timing (COAT) Trial (clinicaltrials.gov NCT01075152), an association was noted between low serum K+ and in-hospital mortality. A standardized electrolyte supplementation protocol was thereafter implemented as a quality improvement initiative. Although electrolyte abnormalities are a common amphotericin toxicity, before February 2011 electrolyte supplementation had been given only as reactive, physician-dependent ad hoc responses to laboratory abnormalities. The objective of this project was to retrospectively assess 3 prospective cryptococcal cohorts: (1) intermittent use of intravenous (IV) fluids and rare ad hoc electrolyte supplementation, (2) standardized IV fluid but rare ad hoc electrolyte supplementation, and (3) standardized administration of IV fluids and universal electrolyte supplementation. Mortality was compared across cohorts to determine whether an aggressive approach to fluid and electrolyte management improves short-term (<30 days) survival after cryptococcal meningitis in persons receiving amphotericin-based therapy to understand how best to safely administer amphotericin in a limited-resource setting. METHODS Study Population Three prospective studies of HIV-infected adults with cryptococcal meningitis were conducted at Mulago Hospital, the national tertiary referral hospital, in Kampala, Uganda. The first cohort enrolled from November 2001 through March 2002, before the availability of antiretroviral therapy (ART), as previously reported [15]. The second cohort enrolled from June 2006 through September 2009, after ART availability. During this time, ART was initiated at a median of 5 weeks [15–17], with additional enrollees from November 2010 to January 2011 from individuals screened for enrollment into the COAT trial (clinicaltrials.gov:NCT01075152) [18]. The third cohort consisted of individuals screened for enrollment into the COAT trial and a follow-on observational cohort that enrolled participants from February 2011 until November 2012. Figure 1 outlines the differences in the clinical management of the 3 cohorts. Inclusion and exclusion criteria are listed in the Supplementary Material, Appendix S1. Written informed consent was obtained. Study protocols were approved by the Institutional Review Boards of Makerere University, the University of Minnesota, and the Uganda National Council of Science and Technology. Figure 1. Graphical explanation of cohort timeline. Timeline outlining the division of cohorts by the years over which each cohort took place, antifungal medications given during induction therapy, IV fluid strategy, and electrolyte supplementation and monitoring strategy. Of note, none of the cohorts included patients between 2003 and 2005 because there was no clinical study of patients with cryptococcal meningitis at Mulago hospital during that time period. Abbreviation: IV, intravenous. Cryptococcal treatment consisted of standardized induction therapy of 14 days of amphotericin B 0.7–1.0 mg/kg in 500 mL 5% dextrose in water over 4 hours in all cohorts. Patient weights were measured in 2001–2002 then estimated until January 2011 when a weighing scale became available again. Lumbar punctures were completed on approximately days 1, 7, and 14 of amphotericin therapy. During 2010–2012 (this included patients from the COAT trial in cohorts 2 and 3), adjunctive oral fluconazole 800 mg/day was also included in the induction regimen [9, 19, 20]. In 2001–2009 (cohorts 1 and 2), after initial induction therapy, consolidation therapy consisted of 8 weeks of fluconazole 400 mg/day. During 2010–2012 (patients from the COAT trial in cohorts 2 and 3), enhanced consolidation therapy began with fluconazole (800 mg/day) until outpatient clinic registration (∼3 additional weeks) and the 14-day cerebrospinal fluid (CSF) culture was known to be sterile, followed by ∼9 additional weeks of fluconazole (400 mg/day) for a 12-week total consolidation. After consolidation therapy, all cohorts received secondary prophylaxis with fluconazole (200 mg/day). Cryptococcus meningitis was diagnosed until April 2011 via latex agglutination and culture. Qualitative cultures were performed in 2001–2002. Quantitative cultures were performed first with a 10 mcL calibrated loop in 2006–2009 [15], and then 100 mcL serial 10-fold dilutions in 2010–2012 [21]. Fluid and Electrolyte Management In 2001–2002 (cohort 1), IV fluids were of limited quantity and intermittently available. In 2006–2009 (cohort 2), all participants received 1 liter of 0.9% NaCl normal saline (NS) before amphotericin, supported via the Minnesota Medical Foundation. In these cohorts, serum electrolyte (Na+, K+) and creatinine monitoring was performed on days 1, 7, and 14, and electrolyte replacement was limited in supply. In 2010–2012 (COAT trial patients in cohorts 2 and 3), participants received 2 liters of NS daily, and after provision of informed consent (median day 5), subjects had additional laboratory safety monitoring with serum electrolyte (Na+, K+, HCO3) and creatinine measurement approximately every 48 hours. In 2010–2012, laboratory results were measured at the Makerere University-Johns Hopkins University (MU-JHU) laboratory using a Roche COBAS Integra 400 Plus Analyzer. The MU-JHU laboratory is a College of American Pathologists-accredited laboratory. In 2001–2009, laboratory tests were performed at the Mulago Hospital laboratory. Electrolyte Supplementation Protocols During the presupplementation period through January 2011 (cohorts 1 and 2), electrolyte management was at the treating physician's discretion, and replacement was generally given in response to abnormal electrolyte levels. Replacement was very infrequently completed. In February 2011, a routine electrolyte supplementation protocol was implemented with K+ and Mg2+ universally given starting on day 1 of amphotericin therapy, data from that point is termed the supplementation period and corresponds with the 2011–2012 cohort (cohort 3). Table 1 summarizes the management differences. The protocol included baseline K+ measurement in addition to the measurements on day 5, 7, 9, 11, and 14 with replacement to goal (K+ = 4.0 mEq/L) after all measurements. Potassium supplementation was 32–40 mEq K+ daily (primarily oral KCl 8 mEq tablets in divided doses). After 1 week of amphotericin, an additional 16 mEq KCl orally was added to baseline supplementation. If hypokalemia occurred despite the universal supplementation, the baseline supplementation was increased by one 8 mEq tablet twice daily in addition to one time replacement doses. The KCl replacement dose was standardized at 10 mEq K+ for each 0.1 mEq/L serum K+ below the target goal (K+ = 4.0 mEq/L). Intravenous replacement was via 40 mEq K+ mixed in 500 mL NS given over 4 hours. Table 1. Electrolyte Management Strategies During Amphotericin Therapy by Time Period Electrolyte Protocol Component Presupplementation (2001–2010) Universal Supplementation (2011–2012) K+ monitoring Day 1, 7, 14(2001–2009)Day 5, 7, 9, 11, 14 (2010) Day 1, 5, 7, 9, 11, 14, and as neededGoal: serum K+ of 4.0 mEq/L K+ supplementation In reaction to laboratory abnormalities Day 1–6: 32–40 mEq KCl daily Day 7–14: 48–56 mEq KCl daily Mild hypokalemia (<3.5 mEq/L): Increase daily routine dose by +16 mEq KCl Replacement of 10 mEq per 0.1 mEq/L deficit to a target of 4.0 mEq/L with each measurement Mg2+ monitoring None None Mg2+ supplementation At physician discretion Day 1–14: 8 mEq Mg2+ dailyMgSO4 5 g IV, if K+ levels <3.0 mEq/L for 3 consecutive days despite adequate KCl replacement and continued supplementation until K+ normalized. Magnesium supplementation was also addressed in the 2011–2012 cohort (cohort 3), although Mg2+ measurement was unavailable on site. Magnesium is wasted with amphotericin use (as noted above), and hypomagnesemia has numerous deleterious effects; however, hypomagnesemia also interferes with the patient's ability to properly replete potassium, thus making magnesium replacement crucial to adequate potassium replacement. Universal supplementation was with magnesium trisilicate (500 mg, 4 mEq) twice daily, which was the only oral Mg2+ locally available, initially. This was later changed to Slow Mag (MgCl) 535 mg (5.33 mEq) tablets for better absorption. In addition, if participants had K+ levels <3.0 mEq/L for 3 consecutive days despite adequate KCl supplementation, participants received 5 g of MgSO4 IV daily until serum K+ levels normalized; thereafter, baseline oral Mg2+ supplementation was continued. Statistical Analysis The primary objective of this analysis was to determine the effect of fluid and electrolyte supplementation on 30-day survival in ART naive patients. A 30-day period was chosen to focus on pre-ART mortality as patients started ART at an average of ∼5 weeks after the diagnosis of cryptococcal meningitis. The 3 cohorts were organized as follows: cohort 1, intermittent IV fluids with no standardized electrolyte management (n = 92 in 2001–2002); cohort 2, universal IV fluids with no standardized electrolyte management (n = 195, with n = 174 in 2006–2009 and n = 21 in November 2010–January 2011; cohort 3, universal IV fluids and universal electrolyte supplementation (n = 142, February 2011–November 2012). Baseline participant characteristics were compared between the cohorts using analysis of variance or 2-sample t tests to compare means and the Fisher exact test to compare frequencies. Serial serum K+ levels were evaluated using a repeated measures model with levels at days 1, 7, and 14 of amphotericin therapy. The occurrence of severe hypokalemia (K+ 2.0–2.4 mEq/L) or mild hypokalemia (K+ 2.5–3.4 mEq/L) was compared with the Fisher exact test. Cox proportional hazard regression compared survival between cohorts with adjustment, when possible, for different baseline characteristics. The survival analysis was restricted to the pre-ART time period only. Participants contributed time from cryptococcal meningitis diagnosis to one of the following: 30-day survival, death, or ART initiation. COAT trial participants randomized to early ART were right-hand censored for the survival analysis at COAT trial randomization (n = 8 in 2010, n = 49 in 2011–2012). Thus, no persons received ART in the 30-day survival analysis, to make all 3 cohorts comparable. For determination of cumulative incidence of hypokalemia, all participants were included. Statistical analysis was conducted using SPSS version 21 (IBM Corporation, Armonk, NY) and evaluated against type I error α < 0.05. RESULTS Patient Characteristics Ninety-two subjects were included in cohort 1, 195 in cohort 2 (174 in 2006–2009 and 21 in November 2010–January 2011), and 142 in cohort 3. Table 2 displays baseline demographics. Demographics were similar, except for the following: proportion of persons with altered mental status with a Glasgow Coma Scale (GCS) < 15 was less in cohort 1 than cohorts 2 and 3 (7.6%, 28%, 31%, P < .001); the mean HIV viral load was slightly higher in cohort 3 than cohort 2 (5.5 vs 5.2 log10 copies/mL, P = .006); and viral load and CD4 counts were not available for cohort 1. Time from hospital admission to definitive diagnosis also improved over time (median 3 to ≤1 days), yet demographics were overall similar. Table 2. Patient Characteristics by Cohort of Persons With Cryptococcal Meningitis in Kampala, Ugandaa Baseline Variables Cohort 1 (2001–02) (n = 92) Limited IV Fluids Cohort 2 (2006–Jan 2011) (n = 195) IV Fluids Cohort 3 (2011–12) (n = 142) IV Fluids and Electrolytes P Value Male 53% 43% 54% .095 Age in years, mean ± SD 35 ± 7 36 ± 9 35 ± 9 .37 CD4 cells/μL, median (IQR) N/A 20 (7–45)b 17 (7–66) .30 HIV viral load, log10 copies/mL, mean ± SD N/A 5.2 ± 0.6b 5.5 ± 0.4 .006 % Glasgow Coma Score <15 7.6% 28%b 31% <.001 CSF opening pressure, cm H2O, mean ± SD 35 ± 14 32 ± 16 29 ± 14 .11 Creatinine at screening, mean ± SD 1.2 ± 0.5 N/A 1.0 ± 0.5 .29 CSF cryptococcal antigen by latex agglutination, geometric mean 1:1600 1:2700 1:2900c .069 Headache duration prior to diagnosis (%) .88 <7 days 34% 30%b 31% 7–14 days 23% 33%b 24% >14 days 43% 37%b 45% Potassium at screening, mEq/L, mean ± SD 4.2 ± 0.8 N/A 3.8 ± 0.6 .010 Potassium at day 7, mEq/L, mean ± SD 4.0 ± 0.7 3.5 ± 1.0d 4.0 ± 0.8 .88 Potassium at day 14, mEq/L, mean ± SD 3.0 ± 0.9 3.0 ± 0.9d 3.6 ± 0.8 .161 Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; IV, intravenous; N/A, data not available; SD, standard deviation. a Baseline participant characteristics were compared between the cohorts using analysis of variance or 2-sample t tests to compare means and the Fisher exact χ2 test to compare frequencies. b Available complete data on 100 patients in the 2006–2009 and 21 in 2010–January 2011 cohort 2. c CRAG latex agglutination titer available on 50 patients from 2011–2012 cohort. d 2010–January 2011 only, n = 16 for day 7, n = 13 for day 14. Incidence of Severe Hypokalemia Figure 2 displays the time to severe or life-threatening (Grade ≥3) hypokalemia (K+ < 2.5 mEq/L) by cohort. The incidence of severe electrolyte abnormalities in cohort 1 during 2001–2002 was negligible. Among those surviving to day 7, only 1.8% (1 of 56) had severe hypokalemia, and zero of 46 who survived to day 14 had severe hypokalemia. Electrolyte data were not prospectively recorded for cohort 2 between 2006 and 2009, but similarly negligible incidence of severe hypokalemia at day 7 and day 14 was recalled (D. B. M., A. M.). However, in the 2010 period of cohort 2 when electrolytes were measured starting at day 5 approximately every 48 hours, the cumulative incidence of severe hypokalemia (K+ < 2.5 mEq/L) was 38% (8 of 21), with 19 severe hypokalemic events occurring in 8 participants in the first 14 days of amphotericin. Severe hypokalemia was evenly distributed between the trial's randomization arms. After the standardized electrolyte protocol was implemented in February 2011, 15 severe hypokalemic events occurred among 12 participants for a cumulative incidence of 8.5% (12 of 142) (relative risk = 4.5; 95% CI, 2.1–9.7; P < .001). Thus, a lack of severe hypokalemia observed in 2001–2009 likely reflects unrecognized severe hypokalemia because of lack of testing between day 8 and 13 as well as a survival bias, namely those who survived to 14 days were more likely the patients without severe hypokalemia during amphotericin therapy. Unintentional missed doses of amphotericin are unlikely because the medication was administered by study nurses and monitored by study physicians. Figure 2. Cumulative incidence of severe hypokalemia (K+ <2.5 meq/L) among 3 cohorts. In 2001–2002 (cohort 1), with minimal electrolyte monitoring on day 7 and 14 only, the detected incidence of severe hypokalemia was 1.1% (1 of 92), being only 1.8% (1 of 56) among those surviving to day 7 and zero of 46 who survived to day 14. In cohort 1, K+ monitoring did not occur between days 8 and 13. In November 2010–January 2011, among COAT trial participants in cohort 2 who received IV fluids and intensive electrolyte monitoring every 48 hours from day 5, the incidence of severe hypokalemia was 38% (8 of 21). After the implementation of universal supplementation (February 21, 2011) and enhanced attention to weight-based dosing of amphotericin (cohort 3), the incidence of severe hypokalemia declined to 8.5% (12 of 142, P < .001 compared to without supplementation). No persons developed clinically significant hyperkalemia with electrolyte supplementation. The majority of the hypokalemia occurs during the second week of amphotericin therapy, thus with minimal monitoring in cohort 1, the lack of detected hypokalemia does not indicate the absence of hypokalemia. Severe hypokalemia rarely occurs before day 7. Without intensive K+ monitoring, absence of hypokalemia at day 14 likely may represent a survival bias. Abbreviation: IV, intravenous. Survival When limited IV fluids were available and intermittent shortages occurred, the 14-day survival was 49% (45 of 92) in 2001–2002. With universal provision of IV fluids in 2006–2010, the 30-day cumulative survival was 62% (log rank P = .003 vs 2001–2002 cohort). In 2011–2012, with universal supplementation of fluids and electrolytes, the 30-day cumulative survival improved to 78% (log rank P = .021 vs 2006–2010 cohort) (Figure 3). Cox regression models were also used to adjust for baseline GCS, creatinine, opening pressure, and potassium: none of these analyses altered the significance of the survival differences. With fluid and electrolyte support, 14-day survival improved by 30% despite similar amphotericin-based treatment (0.7–1.0 mg/kg per day) regimens and frequency of lumbar punctures (median n = 3 in 2001–2002 and in 2011–2012). There was no association between week 1 deaths and hyperkalemia in any cohort. Figure 3. Cumulative survival after cryptococcal meningitis by cohort time period. Fourteen-day survival in 2002 (cohort 1) was 49% (95% confidence interval [CI], 39%–59%), including 8 persons who left against medical advice (presumed dead). In 2006–2010 (cohort 2), with universal IV fluids, the 30-day cumulative survival was 62% (95% CI, 55%–69%; P = .003 vs 2001–2002 cohort). In 2011–2012 (cohort 3), with universal IV fluids and electrolyte supplementation, 30-day cumulative survival improved to 78% (95% CI, 70%–85%; P = .021 vs 2006–2010 cohort, P < .001 vs 2001–2002 cohort). Right-hand censoring occurred at time of antiretroviral therapy (ART) initiation (including n = 8 in 2010; n = 49 in 2011–2012 randomized to early ART; n = 3). DISCUSSION Implementation of a comprehensive electrolyte management protocol was associated with reduced severe hypokalemia and improved survival when added to standard amphotericin treatment and IV fluids for HIV-associated cryptococcal meningitis in a resource-limited setting. Current Infectious Diseases Society of America guidelines for cryptococcal treatment mention that if facilities do not have sufficiently rapid or reliable K+ monitoring, amphotericin use may not be safe [8]. However, further guidance is not provided, and magnesium is not mentioned [8, 22]. In the high-income countries, electrolytes are checked frequently and replaced rapidly in hospitalized patients; however, in the many resource-limited settings, unique barriers exist for the close monitoring of electrolytes. Laboratory facilities may not be reliably available or not able to return results rapidly enough to be acted upon in a clinically useful fashion. Electrolyte replacements themselves may be unavailable in many settings, and cost, although low relative to many other medications, can be a barrier. Thus, although a comprehensive electrolyte management strategy would be ideal, this may not be realistic in all settings. Yet, amphotericin-induced electrolyte wasting is a universal expectation, and so the issue must be addressed. In many illnesses, this may be inconsequential; however, for any illness treated with electrolyte wasting medications, such as amphotericin, K+ and Mg2+ replacement becomes extremely important [23–25]. Stakeholders who influence health policy in low- and middle-income countries should view electrolyte management as part of the package of care for proper cryptococcal treatment. Multiple alternatives to the comprehensive electrolyte replacement strategy detailed above are possible. Amphotericin predictably depletes potassium and magnesium [14, 26]. Thus, scheduled supplementation only without additional measurement or replacement doses would be feasible. Although there is some risk of hyperkalemia due to replacement, the predictable nature of electrolyte wasting allows for scheduled replacement without significant fear of hyperkalemia. Furthermore, because amphotericin's effect on electrolyte wasting is known to be cumulative and dose dependent [11, 14, 26], increasing the potassium supplementation during the second week should be well tolerated. This strategy would reduce overall hypokalemia but likely leave some small percentage of patients (∼10%) severely hypokalemic; moreover, routine replacement would have an acceptable, potential risk of hyperkalemia should even routine monitoring be difficult to obtain. A second strategy would be to use shorter 1-week courses of amphotericin. In a prospective study in Uganda by Muzoora et al [27], 5 days of amphotericin (1.0 mg/kg per day) with adjunctive fluconazole (1200 mg/days) was well tolerated with approximately 75% of the rate of microbiologic clearance (ie, early fungicidal activity) compared to 14 days of amphotericin (1.0 mg/kg per day) but without electrolyte abnormalities. In that study, routine potassium (40 mEq/day) supplementation was given. Similar high levels of efficacy without toxicity were also reported in a similar 7-day randomized trial of amphotericin (1.0 mg/kg per day) with fluconazole (1200 mg/day) in Malawi [28]. An unresolved clinical question, raised by Thomas Harrison and colleagues, St. George's University, London [27, 28], is the ideal length of amphotericin induction, and this question is currently being tested in a phase III trial (ISRCTN45035509). Finally, in resource-limited settings without access to KCl pharmaceutical preparations, foods rich in potassium may be readily available and could provide suitable potassium replacement while on amphotericin. For example, the average US avocado has ∼6.5 mEq of potassium per 100 g, whereas a banana has approximately two thirds of that amount [29]. In a patient able to tolerate food or nasogastric feeding, this may be a reasonable alternative method of potassium supplementation. Defaulting to fluconazole monotherapy to avoid amphotericin toxicity is a poor strategy. The survival with fluconazole monotherapy is ∼30% worse than with amphotericin, in cross-cohort comparisons [10]. Although the lure of less toxicity in fluconazole monotherapy may be appealing, as we have demonstrated here, the electrolyte toxicity of amphotericin is manageable, and acute kidney injury is relatively infrequent (∼8%) with IV fluid prehydration [11]. As mentioned above, in 2001–2002 (cohort 1), IV fluids were of limited quantity and intermittently available. In 2006–2009 (cohort 2), all participants received 1 liter of 0.9% NaCl NS before amphotericin. In 2010–2012 (COAT trial patients in cohorts 2 and 3), participants received 2 liters of NS daily according to trial standard operating procedures. Persistent significant chronic kidney injury is rare: in the 2006–2009 Kampala cohort [17], 95% of survivors had a serum creatinine <2 mg/dL at 5 weeks after cryptococcal meningitis diagnosis, with 80% <1.5 mg/dL. In 2010–2012, 99% of survivors had creatinine <2 mg/dL at 5 weeks. Cost is often considered as a barrier to therapeutic interventions. As an example of the costs of these medications; 1 nonprofit medical wholesaler in Kampala, Uganda supplies the following prices of 1 vial of 10 mEq IV KCl for $1.04 and oral 8 mEq KCl for $3.30 per 100 tablets [30]. Magnesium prices are similar [30]. Although these purchases would require some resources, the cost is quite affordable when one considers the immediate 30-day survival benefit. Supporting local industry and local avocado or banana farmers may be a wiser investment than importing supplies. The main limitation is the historical comparison of 3 cohorts over time. Although fluid and electrolyte management was the major change, other unseen bias influencing mortality may exist. Severity of illness was similar among cohorts and, if anything, increased over time with higher proportions with altered mental status and higher CRAG titers during 2006–2012. Quantitative cultures were performed in cohorts 2 and 3; however, the method was different, and so direct comparison would not be accurate. One of the major improvements in clinical care during the trial of cohort 2 and 3 was improved safety monitoring with more frequent detection of laboratory abnormalities. In 2001–2002, K+ monitoring at day 1, 7, and 14 only detected 2% with hypokalemia at day 7. Yet lack of monitoring did not equate to absence of hypokalemia during the second week of amphotericin, based on a 38% incidence of hypokalemia in 2010 with more frequent monitoring. Likewise, monitoring alone without action did not decrease mortality or hypokalemia in 2010. One might also argue that caregiver or institutional experience may have been gained over time, which explained the survival benefit. However, we believe this explanation is also unlikely given that the medical officers and nurses caring for the patients directly changed with each study (A. K.; D. B. M./A. M.; H. N.). Other specific changes were made. First, the cryptococcal antigen lateral flow assay (Immy, Norman, Oklahoma) was implemented in April 2011 as a point-of-care test to decrease time-to-diagnosis. Although exceedingly helpful, the severity of illness and demographics remained similar. Second, concomitant fluconazole 800 mg/day was given starting in 2010 during induction therapy and as enhanced consolidation until the CSF was known to be sterile. This fluconazole regimen was in place during the initial 2 months of the COAT trial when the investigators detected an association between hypokalemia and mortality. Based on a 2013 trial, the addition of fluconazole to amphotericin did not have a statistical survival benefit over 4 weeks of amphotericin alone [31]. The higher dose fluconazole (800 mg/day) “enhanced” consolidation therapy was used in 2010–2012, but this is of unproven significance in terms of any potential benefit. Although fluconazole adjunctive therapy with amphotericin leads to more rapid clearance of Cryptococcus from the CSF [15, 18], this alone is unlikely to explain the improved survival in the third cohort [31]. The 16% magnitude of 30-day survival difference between cohorts 2 and 3 was beyond the expected effect of added fluconazole (5% better 14-day survival) [31]. A further limitation is the lack of recording of electrolyte and creatinine data during 2006–2009, although the values would be expected to be similar to 2001–2002 or 2010. CONCLUSIONS In summary, survival was substantially improved with IV fluids coupled with universal electrolyte supplementation of K+ and Mg2+, electrolyte monitoring, and standardized electrolyte replacement. WHO Rapid Advice for cryptococcosis treatment published in December 2011 recommended intense monitoring and supplementation based on our earlier data [9, 32]. We believe that the data presented herein should cause stakeholders to emphasize 3 important issues unique to cryptococcal treatment. First, electrolyte management is important for improving survival with amphotericin B deoxycholate therapy. Second, routine, proactive potassium and magnesium supplementation is superior to a reactive approach of replacing electrolytes once a life-threatening deficiency has been identified. Third, more operational research is needed to determine whether potentially shorter courses of 5, 7, or 10 days of amphotericin have a more favorable risk/benefit in resource-limited regions compared to 2 weeks of amphotericin [33]. Using initial quantitative CSF culture burden to guide therapy duration may be more rational than giving all persons 14 days of amphotericin [33]. In conclusion, in comparing 3 cohorts of patients with cryptococcal meningitis treated with amphotericin in Kampala, Uganda, survival significantly improved with a comprehensive electrolyte monitoring and replacement strategy. Supplementary Material Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/). Supplementary Data
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america.
Cryptococcosis is a global invasive mycosis associated with significant morbidity and mortality. These guidelines for its management have been built on the previous Infectious Diseases Society of America guidelines from 2000 and include new sections. There is a discussion of the management of cryptococcal meningoencephalitis in 3 risk groups: (1) human immunodeficiency virus (HIV)-infected individuals, (2) organ transplant recipients, and (3) non-HIV-infected and nontransplant hosts. There are specific recommendations for other unique risk populations, such as children, pregnant women, persons in resource-limited environments, and those with Cryptococcus gattii infection. Recommendations for management also include other sites of infection, including strategies for pulmonary cryptococcosis. Emphasis has been placed on potential complications in management of cryptococcal infection, including increased intracranial pressure, immune reconstitution inflammatory syndrome (IRIS), drug resistance, and cryptococcomas. Three key management principles have been articulated: (1) induction therapy for meningoencephalitis using fungicidal regimens, such as a polyene and flucytosine, followed by suppressive regimens using fluconazole; (2) importance of early recognition and treatment of increased intracranial pressure and/or IRIS; and (3) the use of lipid formulations of amphotericin B regimens in patients with renal impairment. Cryptococcosis remains a challenging management issue, with little new drug development or recent definitive studies. However, if the diagnosis is made early, if clinicians adhere to the basic principles of these guidelines, and if the underlying disease is controlled, then cryptococcosis can be managed successfully in the vast majority of patients.
- Record: found
- Abstract: found
- Article: not found
Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy.
Andrew Kambugu, David B. Meya, Joshua Rhein … (2008)
Cryptococcal meningitis (CM) is the proximate cause of death in 20%-30% of persons with acquired immunodeficiency syndrome in Africa. Two prospective, observational cohorts enrolled human immunodeficiency virus (HIV)-infected, antiretroviral-naive persons with CM in Kampala, Uganda. The first cohort was enrolled in 2001-2002 (n = 92), prior to the availability of highly active antiretroviral therapy (HAART), and the second was enrolled in 2006-2007 (n = 44), when HAART was available. Ugandans presented with prolonged CM symptoms (median duration, 14 days; interquartile range, 7-21 days). The 14-day survival rates were 49% in 2001-2002 and 80% in 2006 (P 200 mm H(2)O). Only 5 patients consented to therapeutic lumbar puncture. There was a trend for higher mortality for pressures >250 mm H(2)O (odds ratio [OR], 2.1; 95% confidence interval [CI], 0.9-5.2; P = .09). Initial CSF WBC counts of <5 cells/mL were associated with failure of CSF sterilization (OR, 17.3; 95% CI, 3.1-94.3; P < .001), and protein levels <35 mg/dL were associated with higher mortality (OR, 2.0; 95% CI, 1.2-3.3; P = .007). Significant CM-associated mortality persists, despite the administration of amphotericin B and HIV therapy, because of the high mortality rate before receipt of HAART and because of immune reconstitution inflammatory syndrome-related complications after HAART initiation. Approaches to increase acceptance of therapeutic lumbar punctures are needed.
- Record: found
- Abstract: found
- Article: not found
Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults.
David Lalloo, Eric Lugada, Jessica Nakiyingi-Miiro … (2002)
Despite the recognition of Cryptococcus neoformans as a major cause of meningitis in HIV-infected adults in sub-Saharan Africa, little is known about the relative importance of this potentially preventable infection as a cause of mortality and suffering in HIV-infected adults in this region. A cohort study of 1372 HIV-1-infected adults, enrolled and followed up between October 1995 and January 1999 at two community clinics in Entebbe, Uganda. Systematic and standardized assessment of illness episodes to describe cryptococcal disease and death rates. Cryptococcal disease was diagnosed in 77 individuals (rate 40.4/1000 person-years) and was associated with 17% of all deaths (77 out of 444) in the cohort. Risk of infection was strongly associated with CD4 T cell counts 100 days in 11% of patients). Survival following diagnosis was poor (median survival 26 days; range 0-138). Cryptococcal infection is an important contributor to mortality and suffering in HIV-infected Ugandans. Improvements in access to effective therapy of established disease are necessary. In addition, prevention strategies, in particular chemoprophylaxis, should be evaluated while awaiting the outcome of initiatives to make antiretroviral therapy more widely available.
Author and article information
Journal
Journal ID (nlm-ta): Open Forum Infect Dis
Journal ID (iso-abbrev): Open Forum Infect Dis
Journal ID (publisher-id): ofid
Journal ID (hwp): ofids
Title:
Open Forum Infectious Diseases
Publisher:
Oxford University Press
ISSN
(Electronic):
2328-8957
Publication date Collection:
September
2014
Publication date
(Electronic):
25
August
2014
Volume: 1
Issue: 2
Electronic Location Identifier: ofu070
Affiliations
Author notes
Correspondence: Nathan C. Bahr, MD, MA, Infectious Diseases Institute, PO Box 22418,
Mulago Hospital Complex, Kampala, Uganda (
bahrx026@
123456umn.edu
).
Article
Publisher ID: ofu070DOI: 10.1093/ofid/ofu070
PMC ID: 4281785
PubMed ID: 25734140
SO-VID: ee3889e6-8cd0-45c1-bc58-b1f78cbfcae1
Copyright © © The Author 2014. Published by Oxford University Press on behalf of the Infectious
Diseases Society of America.
License:
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence ( http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
History
Date
received
: 7
May
2014
Date
accepted
: 10
July
2014
Page count
Pages: 8
Categories
Subject:
Major Articles
Custom metadata
cover-date Summer 2014
Data availability: