- Record: found
- Abstract: found
- Article: not found
Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo
Read this article at
Abstract
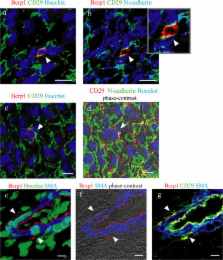
Side population (SP) cells, which can be identified by their ability to exclude Hoechst 33342 dye, are one of the candidates for somatic stem cells. Although bone marrow SP cells are known to be long-term repopulating hematopoietic stem cells, there is little information about the characteristics of cardiac SP cells (CSPs). When cultured CSPs from neonatal rat hearts were treated with oxytocin or trichostatin A, some CSPs expressed cardiac-specific genes and proteins and showed spontaneous beating. When green fluorescent protein–positive CSPs were intravenously infused into adult rats, many more (∼12-fold) CSPs were migrated and homed in injured heart than in normal heart. CSPs in injured heart differentiated into cardiomyocytes, endothelial cells, or smooth muscle cells (4.4%, 6.7%, and 29% of total CSP-derived cells, respectively). These results suggest that CSPs are intrinsic cardiac stem cells and involved in the regeneration of diseased hearts.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche.
- Record: found
- Abstract: found
- Article: not found
Adult cardiac stem cells are multipotent and support myocardial regeneration.
- Record: found
- Abstract: found
- Article: not found
