- Record: found
- Abstract: found
- Article: found
Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences
Read this article at
Abstract
Background:
We investigated the prognostic role of tumour-associated macrophages (TAMs) in patients with head and neck squamous cell carcinoma (HNSCC) treated with definitive chemoradiotherapy (CRT).
Methods:
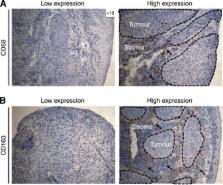
The expression of CD68+, CD163+ and CD11b+ cells was assessed using immunohistochemistry in n=106 pre-treatment tumour biopsy samples and was correlated with clinicopathological characteristics, including T-stage, N-stage, grading, tumour localisation, age and sex as well as local failure-free survival (LFFS), distant metastases-free survival (DMFS), progression-free (PFS), and overall survival (OS). Finally, TAMs expression and vessel density (CD31) were examined in n=12 available early local recurrence samples and compared with their matched primary tumours . The diagnostic images and radiotherapy plans of these 12 patients were also analysed. All local recurrences occurred in the high radiation dose region (⩾70 Gy).
Results:
With a median follow-up of 40 months, OS at 2 years was 60.5%. High CD163 expression in primary tumours was associated with decreased OS ( P=0.010), PFS ( P=0.033), LFFS ( P=0.036) and DMFS ( P=0.038) in multivariate analysis. CD163 demonstrated a strong prognostic value only in human papillomavirus (p16 INK4)-negative patients. Early local recurrence specimens demonstrated a significantly increased infiltration of CD11b+ myeloid cells ( P=0.0097) but decreased CD31-positive vessel density ( P=0.0004) compared with their matched primary samples.
Conclusions:
Altogether, baseline CD163 expression predicts for an unfavourable clinical outcome in HNSCC after definitive CRT. Early local recurrences showed increased infiltration by CD11b+ cells. These data provide important insight on the role of TAMs in mediating response to CRT in patients with HNSCC.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy.
- Record: found
- Abstract: found
- Article: not found