- Record: found
- Abstract: found
- Article: found
Hydrogen Sulfide in Physiology and Pathogenesis of Bacteria and Viruses
Read this article at
Summary
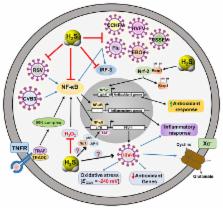
An increasing number of studies have established hydrogen sulfide (H 2S) gas as a major cytoprotectant and redox modulator. Following its discovery, H 2S has been found to have pleiotropic effects on physiology and human health. H 2S acts as a gasotransmitter and exerts its influence on gastrointestinal, neuronal, cardiovascular, respiratory, renal, and hepatic systems. Recent discoveries have clearly indicated the importance of H 2S in regulating vasorelaxation, angiogenesis, apoptosis, ageing, and metabolism. Contrary to studies in higher organisms, the role of H 2S in the pathophysiology of infectious agents such as bacteria and viruses has been less studied. Bacterial and viral infections are often accompanied by changes in the redox physiology of both the host and the pathogen. Emerging studies indicate that bacterial-derived H 2S constitutes a defense system against antibiotics and oxidative stress. The H 2S signaling pathway also seems to interfere with redox-based events affected on infection with viruses. This review aims to summarize recent advances on the emerging role of H 2S gas in the bacterial physiology and viral infections. Such studies have opened up new research avenues exploiting H 2S as a potential therapeutic intervention.
Related collections
Most cited references148
- Record: found
- Abstract: found
- Article: not found
A common mechanism of cellular death induced by bactericidal antibiotics.
- Record: found
- Abstract: found
- Article: not found
Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter?
- Record: found
- Abstract: found
- Article: not found