- Record: found
- Abstract: found
- Article: found
The relationship between the reporting of euphoria events and early treatment responses to pregabalin: an exploratory post-hoc analysis
Abstract
Background
Euphoria is a complex, multifactorial problem that is reported as an adverse event in clinical trials of analgesics including pregabalin. The relationship between the reporting of euphoria events and pregabalin early treatment responses was examined in this exploratory post-hoc analysis.
Methods
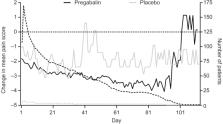
Data were from patients with neuropathic or non-neuropathic chronic pain enrolled in 40 randomized clinical trials, who received pregabalin (75–600 mg/day) or placebo. Reports of treatment-emergent euphoria events were based on the Medical Dictionary of Regulatory Activities preferred term “euphoric mood”. Prevalence rates of euphoria events overall and by indication were assessed. Post-treatment endpoints included ≥30% improvements in pain and sleep scores up to 3 weeks as well as a ≥1-point improvement in daily pain score up to 11 days after treatment.
Results
13,252 patients were analyzed; 8,501 (64.1%) and 4,751 (35.9%) received pregabalin and placebo, respectively. Overall, 1.7% (n=222) of patients reported euphoria events. Among pregabalin-treated patients, a larger proportion who reported euphoria events achieved an early pain response compared with those who did not report euphoria (30% pain responders in week 1 with euphoria events [43.0%], without euphoria events [24.2%]). Results were similar for weeks 2 and 3. For Days 2–11, a larger proportion of pregabalin-treated patients with (relative to without) euphoria events were 1-point pain responders. Findings were similar in pregabalin-treated patients for sleep endpoints (30% sleep responders in week 1 with euphoria events [50.7%], without euphoria events [36.1%]). Similar results were found for weeks 2 and 3. Patients who received placebo showed similar patterns, although the overall number of them who reported euphoria events was small (n=13).
Most cited references48

- Record: found
- Abstract: found
- Article: found
The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care
- Record: found
- Abstract: found
- Article: not found
Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin.
- Record: found
- Abstract: found
- Article: not found