- Record: found
- Abstract: found
- Article: found
Two Cases of Allergy to Insulin in Gestational Diabetes
Read this article at
Abstract
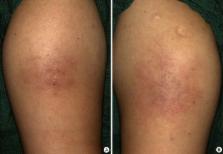
Allergic reaction to insulin is uncommon since the introduction of human recombinant insulin preparations and is more rare in pregnant than non-pregnant females due to altered immune reaction during pregnancy. Herein, we report two cases of allergic reaction to insulin in gestational diabetes that were successfully managed. One case was a 33-year-old female using isophane-neutral protamine Hagedorn human insulin and insulin lispro. She experienced dyspnea, cough, urticaria and itching sensation at the sites of insulin injection immediately after insulin administration. We discontinued insulin therapy and started oral hypoglycemic agents with metformin and glibenclamide. The other case was a 32-year-old female using insulin lispro and insulin detemer. She experienced pruritus and burning sensation and multiple nodules at the sites of insulin injection. We changed the insulin from insulin lispro to insulin aspart. Assessments including immunoglobulin E (IgE), IgG, eosinophil, insulin antibody level and skin biopsy were performed. In the two cases, the symptoms were resolved after changing the insulin to oral agents or other insulin preparations. We report two cases of allergic reaction to human insulin in gestational diabetes due to its rarity.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects.
- Record: found
- Abstract: found
- Article: not found