- Record: found
- Abstract: found
- Article: found
First small-molecule PROTACs for G protein-coupled receptors: inducing α 1A-adrenergic receptor degradation
Read this article at
Abstract
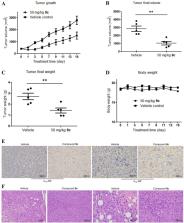
Proteolysis targeting chimeras (PROTACs) are dual-functional hybrid molecules that can selectively recruit an E3 ubiquitin ligase to a target protein to direct the protein into the ubiquitin-proteasome system (UPS), thereby selectively reducing the target protein level by the ubiquitin-proteasome pathway. Nowadays, small-molecule PROTACs are gaining popularity as tools to degrade pathogenic protein. Herein, we present the first small-molecule PROTACs that can induce the α 1A-adrenergic receptor ( α 1A-AR) degradation, which is also the first small-molecule PROTACs for G protein-coupled receptors (GPCRs) to our knowledge. These degradation inducers were developed through conjugation of known α 1-adrenergic receptors ( α 1-ARs) inhibitor prazosin and cereblon (CRBN) ligand pomalidomide through the different linkers. The representative compound 9c is proved to inhibit the proliferation of PC-3 cells and result in tumor growth regression, which highlighted the potential of our study as a new therapeutic strategy for prostate cancer.
Graphical abstract
Small-molecule proteolysis targeting chimeras (PROTACs) of α 1A-adrenergic receptor ( α 1A-AR) were designed and synthesized through conjugation of known α 1-ARs inhibitor prazosin and CRBN ligand pomalidomide through different linkers. Selected compounds displayed remarkable degradation activity. Inhibition of PC-3 cells proliferation and tumor growth regression of these compounds were investigated.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Global estimates of cancer prevalence for 27 sites in the adult population in 2008.
- Record: found
- Abstract: found
- Article: not found
Induced protein degradation: an emerging drug discovery paradigm
- Record: found
- Abstract: found
- Article: not found
