- Record: found
- Abstract: found
- Article: found
The Kappa Opioid Receptor: From Addiction to Depression, and Back
Read this article at
Abstract
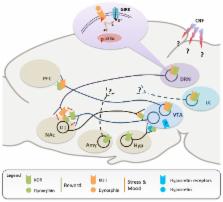
Comorbidity is a major issue in psychiatry that notably associates with more severe symptoms, longer illness duration, and higher service utilization. Therefore, identifying key clusters of comorbidity and exploring the underlying pathophysiological mechanisms represent important steps toward improving mental health care. In the present review, we focus on the frequent association between addiction and depression. In particular, we summarize the large body of evidence from preclinical models indicating that the kappa opioid receptor (KOR), a member of the opioid neuromodulatory system, represents a central player in the regulation of both reward and mood processes. Current data suggest that the KOR modulates overlapping neuronal networks linking brainstem monoaminergic nuclei with forebrain limbic structures. Rewarding properties of both drugs of abuse and natural stimuli, as well as the neurobiological effects of stressful experiences, strongly interact at the level of KOR signaling. In addiction models, activity of the KOR is potentiated by stressors and critically controls drug-seeking and relapse. In depression paradigms, KOR signaling is responsive to a variety of stressors, and mediates despair-like responses. Altogether, the KOR represents a prototypical substrate of comorbidity, whereby life experiences converge upon common brain mechanisms to trigger behavioral dysregulation and increased risk for distinct but interacting psychopathologies.
Related collections
Most cited references151
- Record: found
- Abstract: not found
- Article: not found
Major depressive disorder.
- Record: found
- Abstract: found
- Article: not found
Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons
- Record: found
- Abstract: found
- Article: not found