- Record: found
- Abstract: found
- Article: found
Regulatory Mechanisms of the Wnt/β-Catenin Pathway in Diabetic Cutaneous Ulcers
Read this article at
Abstract
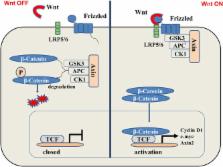
Skin ulcers are a serious complication of diabetes. Diabetic patients suffer from vascular lesions and complications such as peripheral neuritis, peripheral vascular lesions, and collagen abnormalities, which result in skin wounds that are refractory and often develop into chronic ulcers. The healing of skin ulcers requires an inflammatory reaction, wound proliferation, remodeling regulation, and control of stem cells. Studies investigating diabetic cutaneous ulcers have focused on cellular and molecular levels. Diabetes can cause nerve and blood vessel damage, and persistent high blood sugar levels can cause systemic multisite nerve damage based on peripheral neuropathy. The long-term hyperglycemia state enables the polyol glucose metabolism pathway to be activated, increasing the accumulation of toxic substances in the vascular injured nerve tissue cells. Sustained hyperglycemia leads to dysfunction of epithelial cells, leading to a decrease in pro-angiogenic signaling and nitric oxide production. In addition, due to impaired leukocyte function in hyperglycemia, immune function is impaired and the immune response at relevant sites is insufficient, making diabetic foot more difficult to heal. The Wnt/β-catenin pathway is a highly conserved signal transduction pathway involved in a variety of biological processes, such as cell proliferation, apoptosis, and differentiation. It is considered an important pathway involved in the healing of skin wounds. This article summarizes the mechanism of action of the Wnt/β-catenin pathway involved in the inflammatory responses to diabetic ulcers, wound proliferation, wound remodeling, and stem cells. The interactions between the Wnt signal pathway and other metabolic pathways are also discussed.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells.
- Record: found
- Abstract: found
- Article: not found
Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome.
- Record: found
- Abstract: not found
- Article: not found