- Record: found
- Abstract: found
- Article: found
Synergistic Antibacterial Activity and Wound Healing Properties of Selenium-Chitosan-Mupirocin Nanohybrid System: An in Vivo Study on Rat Diabetic Staphylococcus aureus Wound Infection Model
Read this article at
Abstract
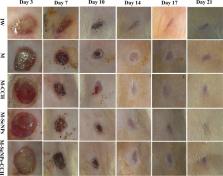
The current study aimed to formulate Selenium-Chitosan-Mupirocin (M-SeNPs-CCH) complex. The nanohybrid system was prepared using chitosan-cetyltrimethylammonium bromide (CTAB)-based hydrogel (CCH) that entrapped mupirocin (M) and selenium nanoparticles (SeNPs). The in vitro studies were performed by evaluation of the antibacterial activity and toxicity on L929 mouse fibroblast cell line. The in vivo study was conducted on rat diabetic wound infection model that was infected by mupirocin-methicillin-resistant Staphylococcus aureus (MMRSA). The wounds were treated by M-SeNPs-CCH nanohybrid system with concentrations of M; 20 mg/ml, CCH; 2 mg/ml and SeNPs; 512 μg/ml in two times/day for 21 days. The therapeutic effect of this nanohybrid system was evaluated by monitoring wound contraction and histopathological changes. Evaluation of the average wound healing time showed a significant difference between the treatment and control groups ( P≤0.05). The histopathological study indicated that the amount of wound healing was considerable in M-SeNPs-CCH nanohybrid system groups compared to the control and M groups. The M-SeNPs-CCH nanohybrid system formulated in this study was able to reduce 3-fold MIC of mupirocin with synergistic antibacterial activity as well as to play a significant role in wound contraction, angiogenesis, fibroblastosis, collagenesis, proliferation of hair follicle, and epidermis growth compared to the control group ( P ≤ 0.05). This research suggests that this nanohybrid system might be a development for the treatment of diabetic wound infection at mild stage.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation.
- Record: found
- Abstract: found
- Article: not found
Biogenic selenium nanoparticles: current status and future prospects.
- Record: found
- Abstract: found
- Article: not found