- Record: found
- Abstract: found
- Article: found
Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease
Read this article at
Abstract
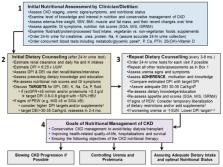
Chronic kidney disease (CKD) affects >10% of the adult population. Each year, approximately 120,000 Americans develop end-stage kidney disease and initiate dialysis, which is costly and associated with functional impairments, worse health-related quality of life, and high early-mortality rates, exceeding 20% in the first year. Recent declarations by the World Kidney Day and the U.S. Government Executive Order seek to implement strategies that reduce the burden of kidney failure by slowing CKD progression and controlling uremia without dialysis. Pragmatic dietary interventions may have a role in improving CKD outcomes and preventing or delaying dialysis initiation. Evidence suggests that a patient-centered plant-dominant low-protein diet (PLADO) of 0.6–0.8 g/kg/day composed of >50% plant-based sources, administered by dietitians trained in non-dialysis CKD care, is promising and consistent with the precision nutrition. The scientific premise of the PLADO stems from the observations that high protein diets with high meat intake not only result in higher cardiovascular disease risk but also higher CKD incidence and faster CKD progression due to increased intraglomerular pressure and glomerular hyperfiltration. Meat intake increases production of nitrogenous end-products, worsens uremia, and may increase the risk of constipation with resulting hyperkalemia from the typical low fiber intake. A plant-dominant, fiber-rich, low-protein diet may lead to favorable alterations in the gut microbiome, which can modulate uremic toxin generation and slow CKD progression, along with reducing cardiovascular risk. PLADO is a heart-healthy, safe, flexible, and feasible diet that could be the centerpiece of a conservative and preservative CKD-management strategy that challenges the prevailing dialysis-centered paradigm.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: not found
A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients.
- Record: found
- Abstract: found
- Article: not found
Functional status of elderly adults before and after initiation of dialysis.
- Record: found
- Abstract: found
- Article: not found