- Record: found
- Abstract: found
- Article: found
Fluocinolone Acetonide 0.19 mg Implant in Patients with Cystoid Macular Edema Due To Irvine–Gass Syndrome
Read this article at
Abstract
Background
Cystoid macular edema (CME) due to Irvine–Gass syndrome (IGS) is one of the common causes of painless visual impairment post-cataract extraction. The treatment of recurrent cases remains unstandardized.
Objective
To evaluate the effectiveness and safety of fluocinolone acetonide intravitreal implant (0.2 µg/day; ILUVIEN ®) in the off-label treatment of recurrent CME due to IGS.
Methods
Retrospective 36-month case series in the Ophthalmology Department of Centro Hospitalar Universitário do Porto, Portugal. Consecutive eyes of patients with recurrent cystoid macular edema due to Irvine–Gass syndrome who underwent a single intravitreal injection of fluocinolone acetonide intravitreal implant were included. Best-corrected visual acuity (logMAR), central macular thickness (µm) and safety (intraocular pressure, mmHg) at baseline and at 6, 12, 24 and 36 months post-administration of the fluocinolone acetonide intravitreal implant were recorded.
Results
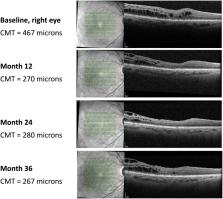
Five eyes from three patients were included. The duration of cystoid macular edema was 67.8±25.9 months and all five eyes received more than 2 intravitreal injections of a corticosteroid (triamcinolone and/or dexamethasone implant) prior to fluocinolone acetonide intravitreal implantation. At baseline (median – interquartile range), best-corrected visual acuity was 0.3–0.3; central macular thickness was 492.0–38.0; and intraocular pressure was 16.0–0. By Month 36, best-corrected visual acuity was 0.4 −0.3; central macular thickness was reduced to 369.0–324.0 and intraocular pressure was 17.0–3.0. Four of five eyes had increased intraocular pressure and were managed with intraocular pressure-lowering eye drops.
Conclusion
We report improved functional and anatomical outcomes after treatment with fluocinolone acetonide intravitreal implant, indicating its use as a therapeutic alternative in recurrent cases of cystoid macular edema due to Irvine–Gass syndrome. Additionally, in eyes with suboptimal response to intravitreal therapies, fluocinolone acetonide intravitreal implant may provide longer recurrence-free periods with reduced treatment burden.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema.
- Record: found
- Abstract: found
- Article: not found
Management of pseudophakic cystoid macular edema.
- Record: found
- Abstract: found
- Article: not found