- Record: found
- Abstract: found
- Article: found
Characterization of photoreceptor degeneration in the rhodopsin P23H transgenic rat line 2 using optical coherence tomography
Read this article at
Abstract
Purpose
To characterize the optical coherence tomography (OCT) appearances of photoreceptor degeneration in the rhodopsin P23H transgenic rat (line 2) in relation to the histological, ultrastructural, and electroretinography (ERG) findings.
Materials and methods
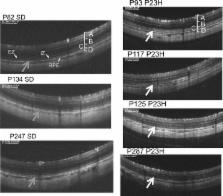
Homozygous rhodopsin P23H transgenic albino rats (line 2, very-slow degeneration model) were employed. Using OCT (Micron IV ®; Phoenix Research Labs, Pleasanton, CA, USA), the natural course of photoreceptor degeneration was recorded from postnatal day (P) 15 to P 287. The OCT images were qualitatively observed by comparing them to histological and ultrastructural findings at P 62 and P 169. In addition, each retinal layer was quantitatively analyzed longitudinally during degeneration, compared it to that observed in wild type Sprague-Dawley (SD) rats. The relationships between the ERG (full-field combined rod-cone response, 3.0 cds/m 2 stimulation) findings and OCT images were also analyzed.
Results
In the qualitative study, the two layers presumably corresponding to the photoreceptor inner segment ellipsoid zone (EZ) and interdigitation zone (IZ) were identified in the P23H rat until PN day 32. However, the photoreceptor inner and outer segment (IS/OS) layer became diffusely hyperreflective on OCT after P 46, and the EZ and IZ zones could no longer be identified on OCT. In contrast, in the SD rats, the EZ and IZ were clearly distinguished until at least P 247. The ultrastructural study showed partial disarrangements of the photoreceptor outer segment discs in the P23H rats at P 62, although a light-microscopic histological study detected almost no abnormality in the outer segment. In the quantitative study, the outer retinal layer including the outer plexiform layer (OPL) and the outer nuclear layer (ONL) became significantly thinner in the P23H rats than in the SD rats after P 71. The thickness of the IS/OS layer was maintained in the P23H rats until P 130, and it became statistically thinner than in the SD rats at P 237. The longitudinal attenuation in the amplitude of the a- and b-waves of ERG was significantly correlated with the thickness of the combined OPL and ONL but not with that of the IS/OS layer.
Conclusion
OCT showed the degenerated photoreceptor IS/OS layer in rhodopsin P23H transgenic rats (line 2) as a diffuse hyperreflective zone, even in the early stage, with the partially disarranged and destabilized OS discs recognizable by ultrastructural assessment but not by a histological study. The amplitude of the a- and b-waves mainly depends on the thickness of the OPL and ONL layer rather than the thickness of the photoreceptor IS/OS layer in P23H rats.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model.
- Record: found
- Abstract: found
- Article: not found
INHERITED RETINAL DYSTROPHY IN THE RAT
- Record: found
- Abstract: found
- Article: not found