- Record: found
- Abstract: found
- Article: found
Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause
Read this article at
Abstract
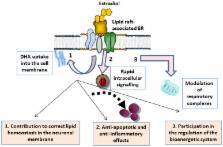
Estrogens (E2) exert a plethora of neuroprotective actions against aged-associated brain diseases, including Alzheimer's disease (AD). Part of these actions takes place through binding to estrogen receptors (ER) embedded in signalosomes, where numerous signaling proteins are clustered. Signalosomes are preferentially located in lipid rafts which are dynamic membrane microstructures characterized by a peculiar lipid composition enriched in gangliosides, saturated fatty acids, cholesterol, and sphingolipids. Rapid E2 interactions with ER-related signalosomes appear to trigger intracellular signaling ultimately leading to the activation of molecular mechanisms against AD. We have previously observed that the reduction of E2 blood levels occurring during menopause induced disruption of ER-signalosomes at frontal cortical brain areas. These molecular changes may reduce neuronal protection activities, as similar ER signalosome derangements were observed in AD brains. The molecular impairments may be associated with changes in the lipid composition of lipid rafts observed in neurons during menopause and AD. These evidences indicate that the changes in lipid raft structure during aging may be at the basis of alterations in the activity of ER and other neuroprotective proteins integrated in these membrane microstructures. Moreover, E2 is a homeostatic modulator of lipid rafts. Recent work has pointed to this relevant aspect of E2 activity to preserve brain integrity, through mechanisms affecting lipid uptake and local biosynthesis in the brain. Some evidences have demonstrated that estrogens and the docosahexaenoic acid (DHA) exert synergistic effects to stabilize brain lipid matrix. DHA is essential to enhance molecular fluidity at the plasma membrane, promoting functional macromolecular interactions in signaling platforms. In support of this, DHA detriment in neuronal lipid rafts has been associated with the most common age-associated neuropathologies, namely AD and Parkinson disease. Altogether, these findings indicate that E2 may participate in brain preservation through a dual membrane-related mechanism. On the one hand, E2 interacting with ER related signalosomes may protect against neurotoxic insults. On the other hand, E2 may exert lipostatic actions to preserve lipid balance in neuronal membrane microdomains. The different aspects of the emerging multifunctional role of estrogens in membrane-related signalosomes will be discussed in this review.
Related collections
Most cited references215
- Record: found
- Abstract: found
- Article: not found
Polyunsaturated fatty acids and their metabolites in brain function and disease.
- Record: found
- Abstract: found
- Article: not found
Estrogen receptors: how do they signal and what are their targets.
- Record: found
- Abstract: found
- Article: not found