- Record: found
- Abstract: found
- Article: found
Effect of Hormone Replacement Therapy on Cardiovascular Outcomes: A Meta-Analysis of Randomized Controlled Trials
Read this article at
Abstract
Background
Hormone replacement therapy (HRT) is widely used to controlling menopausal symptoms and prevent adverse cardiovascular events. However, the benefit and risk of HRT on cardiovascular outcomes remains controversial.
Methodology and Principal Findings
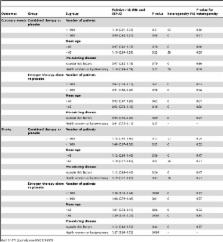
We systematically searched the PubMed, EmBase, and Cochrane Central Register of Controlled Trials databases for obtaining relevant literature. All eligible trials reported on the effects of HRT on cardiovascular outcomes. We did a random effects meta-analysis to obtain summary effect estimates for the clinical outcomes with use of relative risks calculated from the raw data of included trials. Of 1903 identified studies, we included 10 trials reporting data on 38908 postmenopausal women. Overall, we noted that estrogen combined with medroxyprogesterone acetate therapy as compared to placebo had no effect on coronary events (RR, 1.07; 95%CI: 0.91–1.26; P = 0.41), myocardial infarction (RR, 1.09; 95%CI: 0.85–1.41; P = 0.48), stroke (RR, 1.21; 95%CI: 1.00–1.46; P = 0.06), cardiac death (RR, 1.19; 95%CI: 0.91–1.56; P = 0.21), total death (RR, 1.06; 95%CI: 0.81–1.39; P = 0.66), and revascularization (RR, 0.95; 95%CI: 0.83–1.08; P = 0.43). In addition, estrogen therapy alone had no effect on coronary events (RR, 0.93; 95%CI: 0.80–1.08; P = 0.33), myocardial infarction (RR, 0.95; 95%CI: 0.78–1.15; P = 0.57), cardiac death (RR, 0.86; 95%CI: 0.65–1.13; P = 0.27), total mortality (RR, 1.02; 95%CI: 0.89–1.18; P = 0.73), and revascularization (RR, 0.77; 95%CI: 0.45–1.31; P = 0.34), but associated with a 27% increased risk for incident stroke (RR, 1.27; 95%CI: 1.06–1.53; P = 0.01).
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
The interpretation of random-effects meta-analysis in decision models.
- Record: found
- Abstract: found
- Article: not found