- Record: found
- Abstract: found
- Article: found
Glycinergic transmission: glycine transporter GlyT2 in neuronal pathologies
Abstract
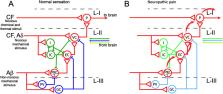
Glycinergic neurons are major contributors to the regulation of neuronal excitability, mainly in caudal areas of the nervous system. These neurons control fluxes of sensory information between the periphery and the CNS and diverse motor activities like locomotion, respiration or vocalization. The phenotype of a glycinergic neuron is determined by the expression of at least two proteins: GlyT2, a plasma membrane transporter of glycine, and VIAAT, a vesicular transporter shared by glycine and GABA. In this article, we review recent advances in understanding the role of GlyT2 in the pathophysiology of inhibitory glycinergic neurotransmission. GlyT2 mutations are associated to decreased glycinergic function that results in a rare movement disease termed hyperekplexia (HPX) or startle disease. In addition, glycinergic neurons control pain transmission in the dorsal spinal cord and their function is reduced in chronic pain states. A moderate inhibition of GlyT2 may potentiate glycinergic inhibition and constitutes an attractive target for pharmacological intervention against these devastating conditions.
Related collections
Most cited references83
- Record: found
- Abstract: found
- Article: not found
SLC6 neurotransmitter transporters: structure, function, and regulation.
- Record: found
- Abstract: found
- Article: not found
GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization.

- Record: found
- Abstract: found
- Article: found