- Record: found
- Abstract: found
- Article: found
Manipulating resident microbiota to enhance regulatory immune function to treat inflammatory bowel diseases
Read this article at
Abstract
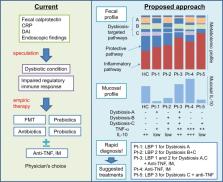
Altered intestinal microbial composition (dysbiosis) and metabolic products activate aggressive mucosal immune responses that mediate inflammatory bowel diseases (IBD). This dysbiosis impairs the function of regulatory immune cells, which normally promote mucosal homeostasis. Normalizing and maintaining regulatory immune cell function by correcting dysbiosis provides a promising approach to treat IBD patients. However, existing microbe-targeted therapies, including antibiotics, prebiotics, probiotics, and fecal microbial transplantation, provide variable outcomes that are not optimal for current clinical application. This review discusses recent progress in understanding the dysbiosis of IBD and the basis for therapeutic restoration of homeostatic immune function by manipulating an individual patient’s microbiota composition and function. We believe that identifying more precise therapeutic targets and developing appropriate rapid diagnostic tools will guide more effective and safer microbe-based induction and maintenance treatments for IBD patients that can be applied in a personalized manner.
Related collections
Most cited references96
- Record: found
- Abstract: found
- Article: not found
Formation of propionate and butyrate by the human colonic microbiota
- Record: found
- Abstract: found
- Article: not found
The microbiome in inflammatory bowel disease: current status and the future ahead.
- Record: found
- Abstract: found
- Article: not found