- Record: found
- Abstract: found
- Article: not found
N-Heterocyclic Olefins as Organocatalysts for Polymerization: Preparation of Well-Defined Poly(propylene oxide)**
Read this article at
Abstract
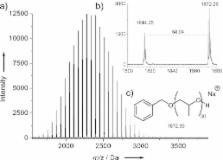
The metal-free polymerization of propylene oxide (PO) using a special class of alkene—N-heterocyclic olefins (NHOs)—as catalysts is described. Manipulation of the chemical structure of the NHO organocatalyst allows for the preparation of the poly(propylene oxide) in high yields with high turnover (TON>2000), which renders this the most active metal-free system for the polymerization of PO reported to date. The resulting polyether displays predictable end groups, molar mass, and a low dispersity ( Đ M<1.09). NHOs with an unsaturated backbone are essential for polymerization to occur, while substitution at the exocyclic carbon atom has an impact on the reaction pathway and ensures the suppression of side reactions.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Optimization of an AMBER Force Field for the Artificial Nucleic Acid, LNA, and Benchmarking with NMR of L(CAAU)
- Record: found
- Abstract: found
- Article: not found
Macromolecular Design via an Organocatalytic, Monomer-Specific and Temperature-Dependent “On/Off Switch”. High Precision Synthesis of Polyester/Polycarbonate Multiblock Copolymers
- Record: found
- Abstract: found
- Article: not found
