- Record: found
- Abstract: found
- Article: found
Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma–COPD overlap
Abstract
Background
Asthma–COPD overlap (ACO) is difficult to diagnose because it is characterized by persistent airflow limitation, and patients present with several manifestations that are usually associated with both asthma and COPD. In this retrospective study, we aimed to evaluate the diagnostic accuracy of fractional exhaled nitric oxide (FeNO) and blood eosinophil counts for the clinical diagnosis of ACO.
Patients and methods
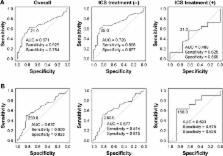
A total of 121 patients were divided into two study groups, COPD alone or ACO, which was based on criteria from the joint document by the Global Initiative for Asthma and the Global initiative for chronic Obstructive Lung Disease. From July 2014 to April 2017, FeNO levels and blood eosinophil counts were measured in specimens from patients naïve to inhaled corticosteroids (ICS) and those using ICS. Receiver operating characteristic curve analysis was used to determine the cutoff values of FeNO and blood eosinophil levels that provided the best differential diagnosis between ACO and COPD.
Results
Among a total of 121 patients, 65 patients were diagnosed with COPD and 56 patients with ACO. The FeNO level was higher in patients with ACO than in patients with COPD (median 24.5 vs 16.0 ppb, respectively; P<0.01). Among patients naïve to ICS, the area under the receiver operating characteristic curve of FeNO values was 0.726, and the optimal diagnostic cutoff level of FeNO was 25.0 ppb, with 60.6% sensitivity and 87.7% specificity for differentiating ACO from COPD. FeNO (≥25.0 ppb) combined with blood eosinophil counts (≥250/μL) showed 96.1% specificity.
Most cited references17

- Record: found
- Abstract: found
- Article: found
The clinical features of the overlap between COPD and asthma
- Record: found
- Abstract: found
- Article: not found
Asthma as a risk factor for COPD in a longitudinal study.
- Record: found
- Abstract: found
- Article: not found