- Record: found
- Abstract: found
- Article: found
Sustained phospholipase C stimulation of H9c2 cardiomyoblasts by vasopressin induces an increase in CDP-diacylglycerol synthase 1 (CDS1) through protein kinase C and cFos
Read this article at
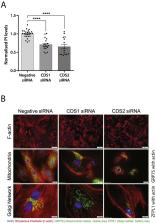
Abstract
Chronic stimulation (24 h) with vasopressin leads to hypertrophy in H9c2 cardiomyoblasts and this is accompanied by continuous activation of phospholipase C. Consequently, vasopressin stimulation leads to a depletion of phosphatidylinositol levels. The substrate for phospholipase C is phosphatidylinositol (4, 5) bisphosphate (PIP 2) and resynthesis of phosphatidylinositol and its subsequent phosphorylation maintains the supply of PIP 2. The resynthesis of PI requires the conversion of phosphatidic acid to CDP-diacylglycerol catalysed by CDP-diacylglycerol synthase (CDS) enzymes. To examine whether the resynthesis of PI is regulated by vasopressin stimulation, we focussed on the CDS enzymes. Three CDS enzymes are present in mammalian cells: CDS1 and CDS2 are integral membrane proteins localised at the endoplasmic reticulum and TAMM41 is a peripheral protein localised in the mitochondria. Vasopressin selectively stimulates an increase CDS1 mRNA that is dependent on protein kinase C, and can be inhibited by the AP-1 inhibitor, T-5224. Vasopressin also stimulates an increase in cFos protein which is inhibited by a protein kinase C inhibitor. We conclude that vasopressin stimulates CDS1 mRNA through phospholipase C, protein kinase C and cFos and provides a potential mechanism for maintenance of phosphatidylinositol levels during long-term phospholipase C signalling.
Graphical abstract
Highlights
-
•
Vasopressin stimulates phospholipase C activity over a 24 hour period leading to cardiac hypertrophy.
-
•
Vasopressin causes a depletion in phosphatidylinositol levels.
-
•
Vasopressin causes an increase in CDP diacylglycerol synthase 1 (CDS1) mRNA which is due to activation of protein kinase C.
-
•
Protein kinase C activates cFos.
-
•
cFos regulates the increase in CDS1 mRNA.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
CHOP is a multifunctional transcription factor in the ER stress response.
- Record: found
- Abstract: found
- Article: not found
The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro.
- Record: found
- Abstract: found
- Article: not found
