- Record: found
- Abstract: found
- Article: found
CTRP-1 levels are related to insulin resistance in pregnancy and gestational diabetes mellitus
Read this article at
Abstract
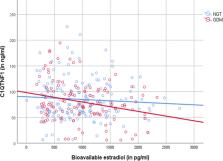
Recent studies have shown higher levels of CTRP-1 (C1QTNF-related protein) in patients with type 2 diabetes compared to controls. We aimed at investigating CTRP-1 in gestational diabetes mellitus (GDM). CTRP-1 levels were investigated in 167 women (93 with normal glucose tolerance (NGT), 74 GDM) of a high-risk population for GDM. GDM was further divided into GDM subtypes depending on a predominant insulin sensitivity issue (GDM-IR) or secretion deficit (GDM-IS). Glucose tolerance was assessed with indices [Matsuda index, Stumvoll first phase index, insulin-secretion-sensitivity-index 2 (ISSI-2), area-under-the-curve (AUC) insulin, AUC glucose] derived from an oral glucose tolerance test (oGTT) performed at < 21 and 24–28 weeks of gestation. In pregnancy, CTRP-1 levels of GDM (76.86 ± 37.81 ng/ml) and NGT (82.2 ± 35.34 ng/ml; p = 0.104) were similar. However, GDM-IR women (65.18 ± 42.18 ng/ml) had significantly lower CTRP-1 levels compared to GDM-IS (85.10 ± 28.14 ng/ml; p = 0.009) and NGT (p = 0.006). CTRP-1 levels correlated negatively with weight, AUC insulin, Stumvoll first phase index, bioavailable estradiol and positively with HbA1c, Matsuda Index and ISSI-2. A multiple regression analysis revealed bioavailable estradiol (β = − 0.280, p = 0.008) and HbA1c (β = 0.238; p = 0.018) as the main variables associated with CTRP-1 in GDM. Postpartum, waist and hip measurements were predictive of CRTP-1 levels instead. CTRP-1 levels were higher postpartum than during pregnancy (91.92 ± 47.27 vs.82.44 ± 38.99 ng/ml; p = 0.013). CTRP-1 is related to insulin resistance in pregnancy and might be a metabolic biomarker for insulin resistance in GDM. CTRP-1 levels were significantly lower during pregnancy than postpartum, probably due to rising insulin resistance during pregnancy.
Related collections
Most cited references43

- Record: found
- Abstract: found
- Article: found
The Pathophysiology of Gestational Diabetes Mellitus
- Record: found
- Abstract: found
- Article: not found