- Record: found
- Abstract: found
- Article: found
Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems
Read this article at
SUMMARY
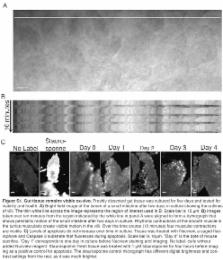
Nonsense mutations that result in the expression of truncated, N-terminal, fragments of the adenomatous polyposis coli (APC) tumour suppressor protein are found in most sporadic and some hereditary colorectal cancers. These mutations can cause tumorigenesis by eliminating β-catenin-binding sites from APC, which leads to upregulation of β-catenin and thereby results in the induction of oncogenes such as MYC. Here we show that, in three distinct experimental model systems, expression of an N-terminal fragment of APC (N-APC) results in loss of directionality, but not speed, of cell motility independently of changes in β-catenin regulation. We developed a system to culture and fluorescently label live pieces of gut tissue to record high-resolution three-dimensional time-lapse movies of cells in situ. This revealed an unexpected complexity of normal gut cell migration, a key process in gut epithelial maintenance, with cells moving with spatial and temporal discontinuity. Quantitative comparison of gut tissue from wild-type mice and APC heterozygotes ( APC Min/+ ; multiple intestinal neoplasia model) demonstrated that cells in precancerous epithelia lack directional preference when moving along the crypt-villus axis. This effect was reproduced in diverse experimental systems: in developing chicken embryos, mesoderm cells expressing N-APC failed to migrate normally; in amoeboid Dictyostelium, which lack endogenous APC, expressing an N-APC fragment maintained cell motility, but the cells failed to perform directional chemotaxis; and multicellular Dictyostelium slug aggregates similarly failed to perform phototaxis. We propose that N-terminal fragments of APC represent a gain-of-function mutation that causes cells within tissue to fail to migrate directionally in response to relevant guidance cues. Consistent with this idea, crypts in histologically normal tissues of APC Min/+ intestines are overpopulated with cells, suggesting that a lack of migration might cause cell accumulation in a precancerous state.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
The intestinal stem cell.
- Record: found
- Abstract: found
- Article: not found