- Record: found
- Abstract: found
- Article: found
Glutathione S-transferase Pi expression predicts response to adjuvant chemotherapy for stage C colon cancer: a matched historical control study
Read this article at
Abstract
Background
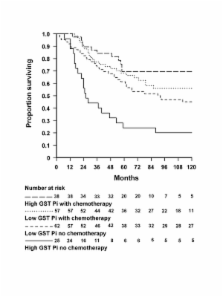
This study examined the association between overall survival and Glutathione S-transferase Pi (GST Pi) expression and genetic polymorphism in stage C colon cancer patients after resection alone versus resection plus 5-fluourouracil-based adjuvant chemotherapy.
Methods
Patients were drawn from a hospital registry of colorectal cancer resections. Those receiving chemotherapy after it was introduced in 1992 were compared with an age and sex matched control group from the preceding period. GST Pi expression was assessed by immunohistochemistry. Overall survival was analysed by the Kaplan-Meier method and Cox regression.
Results
From an initial 104 patients treated with chemotherapy and 104 matched controls, 26 were excluded because of non-informative immunohistochemistry, leaving 95 in the treated group and 87 controls. Survival did not differ significantly among patients with low GST Pi who did or did not receive chemotherapy and those with high GST Pi who received chemotherapy (lowest pair-wise p = 0.11) whereas patients with high GST Pi who did not receive chemotherapy experienced markedly poorer survival than any of the other three groups (all pair-wise p <0.01). This result was unaffected by GST Pi genotype.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Reactive oxygen species in cancer cells: live by the sword, die by the sword.
- Record: found
- Abstract: found
- Article: not found
Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial.
- Record: found
- Abstract: found
- Article: not found