- Record: found
- Abstract: found
- Article: found
Overcoming the Blood–Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours
Abstract
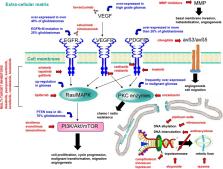
High-grade gliomas are still characterized by a poor prognosis, despite recent advances in surgical treatment. Chemotherapy is currently practiced after surgery, but its efficacy is limited by aspecific toxicity on healthy cells, tumour cell chemoresistance, poor selectivity, and especially by the blood–brain barrier (BBB). Thus, despite the large number of potential drug candidates, the choice of effective chemotherapeutics is still limited to few compounds. Malignant gliomas are characterized by high infiltration and neovascularization, and leaky BBB (the so-called blood–brain tumour barrier); surgical resection is often incomplete, leaving residual cells that are able to migrate and proliferate. Nanocarriers can favour delivery of chemotherapeutics to brain tumours owing to different strategies, including chemical stabilization of the drug in the bloodstream; passive targeting (because of the leaky vascularization at the tumour site); inhibition of drug efflux mechanisms in endothelial and cancer cells; and active targeting by exploiting carriers and receptors overexpressed at the blood–brain tumour barrier. Within this concern, a suitable nanomedicine-based therapy for gliomas should not be limited to cytotoxic agents, but also target the most important pathogenetic mechanisms, including cell differentiation pathways and angiogenesis. Moreover, the combinatorial approach of cell therapy plus nanomedicine strategies can open new therapeutical opportunities. The major part of attempted preclinical approaches on animal models involves active targeting with protein ligands, but, despite encouraging results, a few number of nanomedicines reached clinical trials, and most of them include drug-loaded nanocarriers free of targeting ligands, also because of safety and scalability concerns.
Most cited references174
- Record: found
- Abstract: found
- Article: not found
AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients.
- Record: found
- Abstract: found
- Article: not found
The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype.
- Record: found
- Abstract: found
- Article: not found